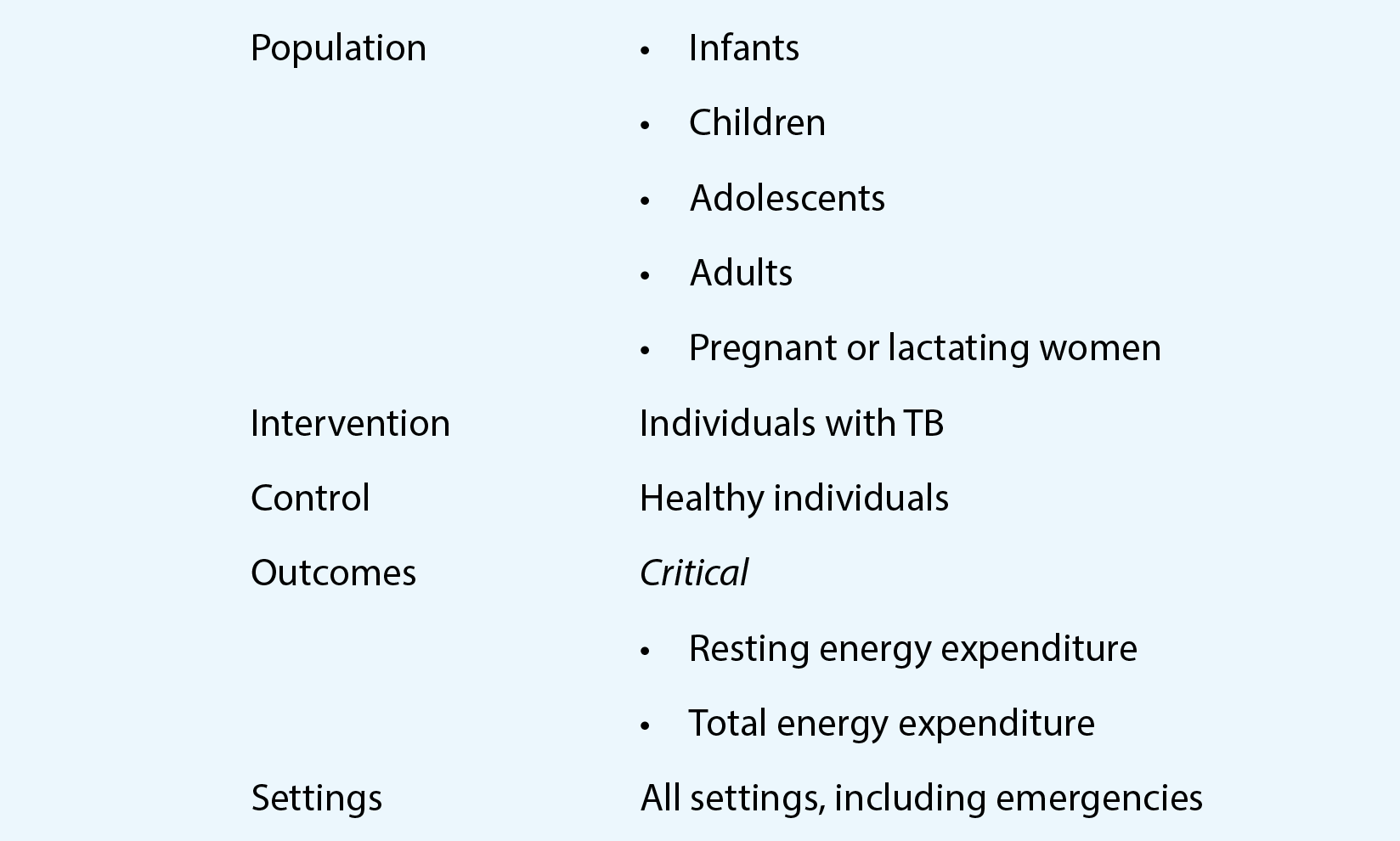
3. Scope of the current update
The WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment include recommendations for the four milestones in the cascade of preventive care, namely identification of risk groups, TB screening and ruling out TB, testing for TBI, and choice and administration of the TPT regimen. The second edition of the TPT guidelines will have the same scope.
 Feedback
Feedback