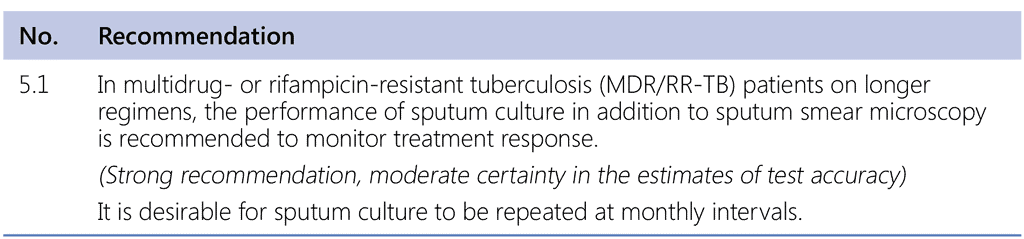
5.2 Justification and evidence
The recommendation in this section addresses the following PICO question:
PICO question (MDR/RR-TB, 2018). In patients with MDR/RR-TB treated with longer or shorter regimens composed in accordance with WHO guidelines, is monitoring using monthly cultures, in addition to smear microscopy, more likely to detect non-response to treatment?
 Feedback
Feedback