Book traversal links for 1.4. Tuberculosis preventive treatment options
TB preventive treatment for an infection with strains presumed to be drug-susceptible can be broadly categorized into two types: monotherapy with isoniazid for at least 6 months (or isoniazid preventive therapy, IPT) and treatment with regimens containing a rifamycin (rifampicin or rifapentine). IPT has been the most widely used form of TB preventive treatment but the shorter duration of rifamycin regimens presents a clear advantage. Preventive treatment for MDR-TB requires a different approach using a fluoroquinolone or other second-line agents. The recommendations for these treatment options, as well as the conditions under which they apply, are discussed in different parts of this section.
17. The following options are recommended for the treatment of LTBI regardless of HIV status: 6 or 9 months of daily isoniazid, or a 3-month regimen of weekly rifapentine plus isoniazid, or a 3 month regimen of daily isoniazid plus rifampicin. (Strong recommendation, moderate to high certainty in the estimates of effect). A 1-month regimen of daily rifapentine plus isoniazid or 4 months of daily rifampicin alone may also be offered as alternatives. (Conditional recommendation, low to moderate certainty in the estimates of effect).
18. In settings with high TB transmission, adults and adolescents living with HIV who have an unknown or a positive LTBI test and are unlikely to have active TB disease should receive at least 36 months of daily isoniazid preventive treatment (IPT). Daily IPT for 36 months should be given whether or not the person is on ART, and irrespective of the degree of immunosuppression, history of previous TB treatment and pregnancy in settings considered to have a high TB transmission as defined by national authorities. (Conditional recommendation, low certainty in the estimates of effect)
Regimens containing isoniazid or rifamycins
Both recommendations already featured in WHO guidance from 2015 (13),(25). A strong recommendation for TB preventive treatment alternatives to 6H, based on evidence of low to high certainty, featured in previous WHO guidance (12),(13),(16). In 2019 the GDG made edits to the text of this recommendation to add the two new conditional recommendations for daily rifapentine plus isoniazid for 1 month (1HP) and daily rifampicin monotherapy for 4 months (4R) in all settings. These new recommendations are based, respectively, on low to moderate certainty in the estimates of effect. In addition, instead of a previous range of 3–4 months, the GDG now recommends a duration of 3 months for daily isoniazid plus rifampicin (3HR) and of 4 months for daily rifampicin alone (4R) to reflect the usual length of time for which these regimens are currently employed. Moreover, three previous recommendations on the use of 6H, 3HR in people <15 years and 3HP in high TB prevalence settings that featured separately in previous guidance are now proposed as alternative options. The revised recommendation makes all LTBI options applicable to all settings.
Justification and evidence
Daily isoniazid monotherapy
The efficacy of daily isoniazid monotherapy for six months (6H) or more in different populations and settings has been shown in a number of systematic reviews (18),(66),(67). A systematic review of RCTs in People with HIV showed isoniazid monotherapy reduces the overall risk for TB by 33% (RR 0.67; 95% CI 0.51; 0.87), and the that preventive efficacy reached 64% for people with a positive TST (RR 0.36; 95% CI 0.22; 0.61) (18). Furthermore, the efficacy of the 6-month regimen was not significantly different from that of 12 months’ daily isoniazid monotherapy (RR 0.58; 95% CI 0.3; 1.12). A recent systematic review of RCTs also showed a significantly greater reduction in TB incidence among participants given the 6-month regimen than in those given a placebo (Odds ratio [OR] 0.65; 95% CI 0.50; 0.83)(68). No controlled clinical trials were found of daily isoniazid monotherapy for 9 months (9H) versus 6H. Re-analysis and modelling of the United States Public Health Service trials of isoniazid conducted in the 1950s and 1960s, however, showed that the benefit of isoniazid increases progressively when it is given for up to 9–10 months and stabilizes thereafter (69). For this reason, 9H is retained as an alternative regimen to 6H in the recommended TB preventive treatment options.
Regarding the second recommendation above, a systematic review and meta-analysis of three RCTs of People with HIV in settings with high TB prevalence and transmission showed that continuous IPT can reduce the risk for active TB by 38% more than 6 months’ isoniazid (70). The effect was greater in people with a positive TST (49% for active TB and 50% for death). In those with a negative TST, neither effect was significant, although the point estimate indicated a reduction in TB incidence of 27%. In two of the studies reviewed ART was not used and in the third ART coverage was low at baseline but increased during the period of observation.
Daily rifampicin plus isoniazid for 3 months (3HR)
A systematic review updated in 2017 showed that the efficacy and the safety profile of 3–4 months’ daily rifampicin plus isoniazid were similar to those of 6 months’ isoniazid (68),(71). A previous GDG therefore strongly recommended that daily rifampicin plus isoniazid could be used as an alternative to isoniazid in settings with a TB incidence <100 / 100,000 population (13). A new review to compare the effectiveness of rifampicin plus isoniazid daily for 3 months with isoniazid for 6 or 9 months in children identified one RCT and two observational studies (72),(73),(74) (see also GRADE evidence summaries for PICO 5 in Annex 2). The RCT (73) reported no clinical disease in either group and used new radiographic findings suggestive of active TB as a proxy for clinical disease. Fewer participants given daily rifampicin plus isoniazid than those given 9 months of isoniazid developed radiographic changes (RR 0.49, 95% CI 0.32; 0.76). The authors also reported a lower risk for adverse events (RR 0.33, 95% CI 0.20; 0.56) and a higher adherence rate (RR 1.07, 95% CI 1.01; 1.14) among children given daily rifampicin plus isoniazid. Similar findings were reported in the observational studies (72),(74).
Daily rifampicin monotherapy for 4 months (4R)
A previous systematic review conducted for the 2015 LTBI guidelines and updated in 2017, found similar efficacy for 3–4 months’ daily rifampicin and 6H (odds ratio, 0.78; 95% CI, 0.41;1.46) (68),(71). The review also showed that individuals given rifampicin daily for 3–4 months had a lower risk for hepatotoxicity than those treated with isoniazid monotherapy (OR 0.03; 95% CI 0.00;0.48).
In 2019, the GDG discussed the implications of using 4R in high TB burden settings based on findings from RCTs of 4R vs 9H that included adults and children from such countries(75),(76),(77),(78). In study participants >17 years, the difference in rate of confirmed TB between 4R and 9H (4R arm minus 9H arm) was <0.01 cases per 100 person-years (95%CI, −0.14; 0.16); the difference in treatment completion was 15.1% (95% CI, 12.7; 17.4); the difference for Grade 3–5 adverse events was −1.1% (95% CI, −1.9; -0.4). In individuals <18 years, the difference in rate of active TB between 4R and 9H was -0.37 cases per 100 person-years (95% CI, −0.88; 0.14); the difference in treatment completion was 13.4% (95% CI, 7.5; 19.3); the difference in risk for adverse events attributed to the medicine used and resulting in discontinuation was −0.0 (95% CI, −0.1; 0.1). The evidence underpinning this revised recommendation is summarised in the GRADE tables for PICO 6 in Annexes 2 and 3.
Daily rifapentine plus isoniazid for 1 month (1HP)
In 2019, the GDG considered data from the only known published study of the 1HP regimen: a randomized, open-label, phase 3 non-inferiority trial comparing the efficacy and safety of 1HP with 9 months of isoniazid alone (“9H”) in People with HIV who were in areas of high tuberculosis prevalence or who had evidence of LTBI (79). Enrolment was restricted to individuals ≥13 years old who were not pregnant or breastfeeding. Noninferiority would be shown if the upper limit of the 95% confidence interval for the between-group difference in the number of events per 100 person-years was less than 1.25. Among all study participants, the difference in incidence rate of TB (including deaths from any cause) between 1HP and 9H (i.e. 1HP arm minus 9H arm) was −0.02 per 100 person-years (95% confidence interval [CI], −0.35; +0.30); the relative risk (RR) for treatment completion of 1HP over 9H was 1.04 (95% CI, 0.99; 1.10); the RR for Grade 3–5 adverse events was 0.86 (95% CI, 0.58; 1.27); hazard ratio of death from any cause was 0.75 in favour of 1HP (95% CI, 0.42; 1.31); RR for emergence of resistance to isoniazid and rifampicin were, respectively, 1.63 (95% CI, 0.17; 15.99) and 0.81 (95% CI, 0.06; 11.77). Overall non-inferiority as defined by the study protocol was thus shown in the modified intention to treat (mITT) population. Non-inferiority was also shown for the sub-group with confirmed LTBI infection (incidence rate difference per 100 person-years = 0.069 [-0.830 to 0.690]), as well as in males and females, and among those on or without ART at start of study. The number of patients with a CD4+ <250 cells per cu mm was small, and neither inferiority or noninferiority of 1HP was shown in this stratum. The evidence underpinning this new recommendation is summarised in the GRADE tables for PICO 7 in Annexes 2 and 3.
Weekly rifapentine plus isoniazid for 3 months (3HP)
A systematic review was conducted for the 2018 guidelines update to compare the effectiveness of a 3-month weekly regimen of rifapentine plus isoniazid (3HP) with that of isoniazid monotherapy. The review covered four RCTs (80),(81),(82),(83), which were analysed for three subgroups: adults with HIV infection, adults without HIV infection and children and adolescents, who could not be stratified according to HIV status because the relevant studies were lacking. The evidence underpinning this revised recommendation is summarised in the GRADE tables for PICO 8 in Annexes 2 and 3.
Two of the RCTs involved adults with HIV from South Africa, Peru and a number of countries with a TB incidence <100 / 100,000 population. No significant difference was found in the incidence of active TB between participants given a 3HP and 6H or 9H (RR 0.73, 95% CI 0.23; 2.30). Furthermore, the risk for hepatotoxicity was significantly lower with 3HP in adult People with HIV (RR 0.26, 95% CI 0.12; 0.55) and in those without HIV (RR 0.16, 95% CI 0.10; 0.27). The 3HP regimen was also associated with a higher completion rate in all subgroups (adults with HIV: RR 1.25, 95% CI 1.01; 1.55; adults without HIV: RR 1.19, 95% CI 1.16; 1.22; children and adolescents: RR 1.09, 95% CI 1.03; 1.15). One RCT included a comparison between 3HP and continuous isoniazid monotherapy in adult People with HIV (80). No significant difference in TB incidence was found in an intention-to-treat analysis; however, a per-protocol analysis showed a lower rate of TB infection or death in participants given continuous isoniazid. In all the studies, 3HP was given under direct observation. In a study of 3HP in 112 pregnant women, the rates of spontaneous abortion and birth defects were similar to those in the general US population (84).
Implementation considerations
The decision on which treatment to offer should not be confined to the manner in which it was studied in a trial (e.g. 1HP to replace 9H). The GDG agreed that the benefits of all the treatment options being recommended outweigh the potential harm. The programmes and clinicians should also consider the characteristics of the individual concerned to maximise the likelihood that treatment is completed as expected. Regimen choice is determined by considerations such as age, risk of toxicity or interaction, co-morbidity, drug susceptibility of the strain of the most likely source case, availability and the individual’s preferences.
On the basis of existing practice, albeit in the absence of a direct comparison, the GDG judged that 9H is an equivalent option to 6H in countries with a strong health infrastructure. It noted, however, that 6H is preferable to 9H from the point of view of feasibility, resource requirements and acceptability to patients.
All recommended treatment options are possible in People with HIV. The recommendation to give at least 36 months of daily isoniazid monotherapy in People with HIV in high TB transmission settings is conditional and based on evidence that longer-term IPT significantly adds benefit to ART. The efficacy, safety and convenience of repeated treatment with shorter rifapentine regimens is being studied in People with HIV in such settings. The definition of a high TB transmission setting should be established by the national authorities (see also Definitions). Testing for LTBI is not a prerequisite for TB preventive treatment in People with HIV but its use is encouraged because people who are TST positive have a greater protective benefit from TB preventive treatment. People with HIV with a negative TST should not receive 36 months of daily IPT.
The GDG agreed unanimously that the benefits of 3HR for infants and children < 15 years of age outweigh the harm, given its safety profile, the higher rate of completion as compared with isoniazid monotherapy and the availability of child-friendly, fixed-dose combinations of rifampicin and isoniazid.
The GDG therefore made a strong recommendation despite the low quality of the evidence. There are no or very limited data on the performance and pharmacology of rifapentine in children < 2 years. The 3HP regimen is only recommended for use in children aged 2 years and more while the 1HP regimen in individuals aged 13 years and more.
The 2019 GDG considered that there was moderate certainty that 4R is not inferior to 9H, and when also considering the good safety profile of the 4R regimen and its reduced length, it recommended that this regimen may also be used in high TB-burden settings. When deciding to make a conditional recommendation the GDG considered that most people would value a shorter regimen, but raised concerns regarding variability in acceptability, uncertainty in resource requirements given its higher cost, and potential for reducing equity should it deflect resources and decrease treatment coverage of more vulnerable individuals. The GDG agreed that the introduction of 4R needs to be accompanied by mobilization of appropriate resources from the start to avoid shortages in other programmatic needs. The GDG also observed that impact on equity could change if the price and policy of use of 4R also change (see also Annex 3 for more details on the GDG decisions).
With respect to 1HP, the 2019 GDG concluded that there was low certainty that its effectiveness would be non-inferior to 9H when used under programmatic settings in different populations at risk. When taking also into account the good safety profile of 1HP and its much shorter length when compared with other approved LTBI regimens, the GDG recommended that this regimen may also be used in high TB-burden settings and in people without HIV infection. The GDG considered that most people would value its much shorter duration than other options, that its implementation would be feasible, but raised concerns regarding uncertainty in resources requirements and the potential for reducing equity, leading to a conditional recommendation (see also Annex 3 for more details on the GDG decisions).
In the current update, the GDG considered that all regimens could be used in any setting, regardless of TB burden, provided that the health infrastructure can ensure the treatment is given correctly without creating inequities, and that active TB can be excluded reliably before the initiation of treatment.
The GDG noted that all the treatment options can be self-administered. An RCT showed that self-administered treatment of the 3HP is not inferior to directly observed treatment (85); however, there is little further evidence on self-administration of this regimen. The GDG noted that a requirement for a direct observation could be a significant barrier to the implementation. People receiving TB preventive treatment should also be supported through access to advice on treatment and management of adverse events at their encounters with the health services. The GDG further noted that individuals receiving treatment, clinicians providing treatment and programme managers would prefer shorter to longer regimens.
Drug-drug interactions
Rifamycins induce certain cytochrome P-450 enzymes and may therefore interfere with medicines that depend on this metabolic pathway, accelerating their elimination. These include ART as well as many other medicines such as anticonvulsants, antiarrhythmics, quinine, oral anticoagulants, antifungals, oral or injectable contraceptives, corticosteroids, cyclosporine, fluoroquinolones and other antimicrobials, oral hypoglycaemic agents, methadone, and tricyclic antidepressants. Such medicines may therefore need to be avoided when rifampicin or rifapentine containing regimens are given, or that their dosages are adjusted.
Regimens containing rifamycins should be prescribed with caution to People with HIV who are on ART because of potential drug–drug interactions. These regimens should not be administered to people receiving protease inhibitors or nevirapine, including HIV-exposed infants on preventive treatment. Rifampicin can decrease the concentrations of other antiviral agents: atazanavir, darunavir, fosamprenavir, lopinavir, saquinavir and tipranavir. It should not be used with saquinavir/ritonavir. No dose adjustment is required when rifampicin is co-administered with efavirenz. The dose of dolutegravir however needs to be increased to 50 mg twice daily when given together with rifampicin (86), a dose that is usually well tolerated and gives equivalent efficacy in viral suppression and recovery of CD4 cell count compared with efavirenz.
The 3HP regimen can be administered to patients receiving efavirenz-based antiretroviral regimens without dose adjustment, according to a study of pharmacokinetics (87). Administration of rifapentine with raltegravir was found to be safe and well tolerated (88). A drug interaction study in healthy volunteers of dolutegravir with once weekly HP reported toxicities in 2 of 4 participants (89). However results released more recently from a Phase 1/2 trial of 3HP and dolutegravir in adults with HIV reported good tolerance and viral load suppression, no adverse events of Grade >3 related to the HP, and did not indicate that rifapentine reduced dolutegravir levels sufficiently to require dose adjustment (90). The GDG stressed however the continued need for studies of the pharmacokinetics of 3HP concomitantly with other medicines, particularly ART.
Concurrent use of alcohol needs to be avoided with TB preventive treatment.
Pregnancy
In preparation for the current update, a systematic review was conducted in 2019 to assess evidence in support or against recent reports from one RCT of adverse pregnancy outcomes associated with the use of IPT (91),(92). In addition to this RCT, three non-randomized, comparative observational studies provided data on at least one of the pregnancy outcomes in women with HIV (93),(94),(95) (see PICO 9 in Annex 2). While the RCT showed a higher risk of adverse pregnancy outcomes in women who initiated IPT during pregnancy (Mantel-Haenszel OR stratified by gestational age, 1.51 95%CI 1.09; 2.10), all three other studies reported an overall OR <1 suggesting the opposite (I2 =80%, p=0.002). A meta-analysis from two observational studies that cited adjusted estimates and whose data could be pooled suggested lower risk for composite adverse pregnancy outcomes (OR 0.40, 95%CI 0.20; 0.74) (93),(94). The observational studies did not reproduce the associations with IPT reported by the RCT for individual adverse outcomes such as foetal/neonatal death, prematurity, low birth weight, and congenital anomaly. No statistically significant risks for maternal hepatotoxicity, Grade 3 or 4 events or death were reported by any of the four studies. Based upon these findings the GDG concluded that there were insufficient grounds to change previous guidance or to develop a separate recommendation for the use of IPT in pregnant women with HIV. The GDG considered that systematic deferral of IPT to the postpartum would deprive women from its protective effect at a point when they are more vulnerable to TB. Appropriate care during the antenatal and postnatal periods and during delivery may reduce risk of adverse pregnancy outcome. While obtaining baseline liver function tests when IPT is given in pregnancy is strongly encouraged when feasible, it is not required, and routine liver function testing when IPT is given in pregnancy is not indicated unless there are other risk factors for liver toxicity are present. Vitamin B6 supplementation should however be considered. The GDG agreed that this is an area requiring more research, such as on pharmacokinetics and pharmacovigilance of IPT and other preventive treatment regimens. Rifampicin is generally considered safe in pregnancy. There are limited data on the pharmacokinetics and safety of rifapentine in pregnancy and therefore the use of 1HP in pregnancy would best await more data to ensure appropriate dosing and at least preliminary safety data for this regimen in pregnant women.
Other subgroups and settings
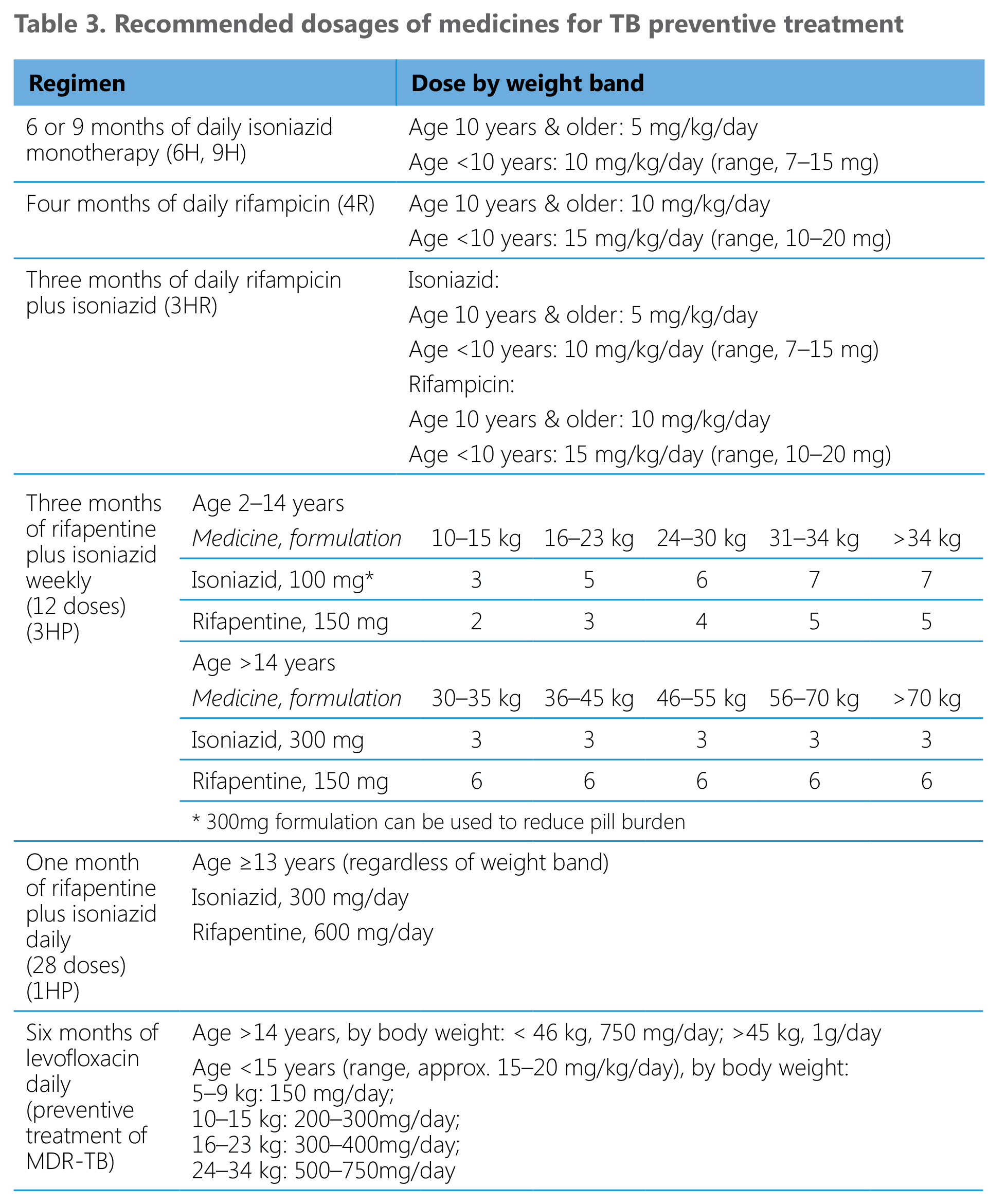
The recommended dosages for TB preventive treatment regimens in adults and children are shown in Table 3. Regimens based on isoniazid and rifampicin can be used in individuals of all ages. There are no or very limited data on the efficacy and safety of rifapentine in children < 2 years and the 3HP regimen is only recommended for use in children aged 2 years and more. The data from the 1HP trial relates only to individuals aged 13 years and more. The GDG considered that extrapolation of effects to children aged 2–12 years is reasonable, although the dosage of daily rifapentine in this age group has yet to be established. The suitability of this regimen in people <13 years needs to be reviewed once results from studies of pharmacokinetics and safety in children of all ages become available in a near future.
In candidates for transplantation or anti-TNF treatment it may be particularly important to complete TB preventive treatment fast and therefore shorter regimens like 1HP and 3HP could have an advantage over longer treatments. Likewise, in homeless people and in people being released from prison, in whom there is limited opportunity for repeated encounters during treatment, shorter treatment could be more suitable than longer regimens.
In addition to People with HIV on ART, other populations who may be more commonly at risk of drug-drug interactions from rifampicin include women of childbearing age on contraceptive medicines (who need to be counselled about potential interactions and consider nonhormonal birth control while receiving rifampicin) and opiate users on substitution therapy with methadone.
Contacts of patients with laboratory confirmed isoniazid-resistant, rifampicin-susceptible TB (Hr-TB) may be offered a four-month regimen of daily rifampicin.
Other considerations
Given the widespread use of rifampicin-containing fixed dose combinations to treat drug-susceptible TB, single dose rifampicin has become less available to disease programmes. If the 4R regimen will be used more often the demand for loose tablets of rifampicin will increase and programmes would need to procure it. Quality-assured supplies of rifampicin should be used. The provision of 4R outside the TB programme centres (e.g. primary care facilities, HIV programmes) should be accompanied by stepwise guidance on how to maximise the effect of rifampicin and avoid it being diverted for use as a broad-spectrum antibiotic.
Fixed-dose combinations (FDC) of HR should be used where possible to reduce the number of pills to be taken. FDCs of 3HP are expected to be released in a near future and will facilitate administration. Shorter regimens are also more likely to be completed. Concerns about adherence should not be a barrier to starting TB preventive treatment and support provided to enable better person-centred care. No data-supported recommendations exist on how to handle interruptions of TB preventive treatment, i.e. how many missed doses can be made up for by prolonging treatment without compromising efficacy?
Individuals at risk for peripheral neuropathy, such as those with malnutrition, chronic alcohol dependence, HIV infection, renal failure or diabetes, or who are pregnant or breastfeeding, should receive pyridoxine (vitamin B6) when taking isoniazid-containing regimens. A lowering of isoniazid dosage from the one proposed may be required to avoid toxicity if there is a high population prevalence of “slow acetylators”. Combination tablets of co-trimoxazole, isoniazid and pyridoxine could be helpful in People with HIV. However, unavailability of pyridoxine should not be a reason to withhold TB preventive treatment.
Interventions to enhance adherence and completion of treatment should be tailored to the specific needs of risk groups and the local context. A systematic review conducted for the WHO 2015 LTBI guidelines provided heterogeneous results for interventions to improve treatment adherence and completion, and the evidence was considered inconclusive (14). The WHO guidelines for treatment of drug-susceptible active TB propose several interventions to support adherence, which could also be applied to TB preventive treatment (96).
In areas with high background resistance to rifampicin, such as countries in eastern Europe, it is particularly important to try to get the strain from the presumed source tested for drug susceptibility so that treatment given is more likely to work. If there is rifampicin monoresistance or other contraindications to rifampicin, then an isoniazid regimen of 6 or more months may be the most appropriate option. Unfortunately, in many settings, rifampicin resistance is often accompanied by isoniazid resistance – multidrug-resistant TB (MDR-TB) – requiring different preventive medication (see below).
Preventive treatment for MDR-TB
Justification and evidence
Evidence for effectiveness and safety of MDR-TB preventive treatment was reviewed and summarised in Section 1.1. The medicines used in these studies were mainly fluoroquinolones (e.g. moxifloxacin, levofloxacin) with or without other agents (e.g. ethambutol, ethionamide). The median proportion of participants who discontinued treatment because of adverse events in all the studies was 5.1% (interquartile range, 1.9–30.2%).
While ethambutol is considered safe in pregnancy, ethionamide was associated with teratogenic potential at high doses in preclinical animal studies, with minimal data in human pregnancy. Although there has been concern about the use of fluoroquinolones in children because of retardation of cartilage development shown in animals (97), similar effects have not been demonstrated in humans (98),(99). While the effects of fluoroquinolones on bone and cartilage in animals have not been observed in humans, available data and infant follow-up times are limited. One meta-analysis of observational studies including 2800 pregnant women exposed to fluoroquinolones found no differences in birth defects, spontaneous abortion or prematurity compared to unexposed pregnant women (100). Recent alerts have however highlighted the safety concerns associated with prolonged use of fluoroquinolones in humans (101),(102).
There is limited evidence for the optimal duration of MDR-TB preventive treatment, and this should be based on clinical judgement. Regimens used in the studies conducted so far were given for 6, 9 and 12 months. None of studies included data on pharmacokinetics and safety in pregnancy or a comparison of the risk for adverse events, although one reported that no serious adverse events could be attributed to fluoroquinolone-based preventive treatment (36).
Implementation considerations
The regimen of preventive treatment of MDR-TB contacts should be individualized and based on reliable information on the drug resistance profile of the presumed source. Later-generation fluoroquinolones (e.g. levofloxacin or moxifloxacin) may be used unless the strain of the presumed source shows resistance to these medicines. A dosing schedule for levofloxacin in children and adults is proposed in Table 3. Paediatric formulations of levofloxacin can be used for this purpose. For strains showing additional resistance other treatment regimens used in some of the studies may be used (37).
Contacts of people with rifampicin-resistant TB (RR-TB) are usually treated as for MDR-TB unless isoniazid-susceptibility in the index case is reliably confirmed, in which case IPT may be effective.
As the recommendation for preventive treatment in MDR-TB exposure is based on very low-quality evidence, people must be given detailed information about the potential benefits and harms of giving fluoroquinolones or other regimens. In view of uncertainties about the balance of benefit to harm, informed consent, preferably in writing, is required, based on the local context and practices in similar situations.