Consolidated Guidelines
A5.4 A survey to explore the programmatic feasibility of levofloxacin (Lfx) TPT for MDR-TB contacts11
Introduction
The survey assessed the feasibility of programmatic use of Lfx for TPT among contacts of MDR-TB patients in the eventuality of a WHO recommendation for its programmatic use. The objective was to collect perspectives from national TB programmes (NTPs), explore current practices for MDR TPT, its programmatic feasibility, affordability, impact on equity, acceptability to patients and health-care workers and to inform the discussion of WHO Guideline group at its meeting on 4–6December 2023.
Methods
A5.3 Assessing fluoroquinolone (levofloxacin) acceptability among contacts of MDR-TB patients: a qualitative study10
Objective: to assess the values, preferences, acceptability and feasibility of Lfx as TPT for adult HHCs of patients diagnosed with MDR-TB in five low- and middle-income countries: Georgia, India, Indonesia, South Africa and Viet Nam.
A5.2 Use of fluoroquinolones for TB preventive treatment in contacts of persons with MDR-/RR-TB: A systematic review
Harsimren Sidhu, Siobhan Carroll, Dick Menzies9
Introduction
A5.1 Summary of TB CHAMP and V-QUIN clinical trials
Tuberculosis Child Multidrug-Resistant Preventive Therapy Trial (TB CHAMP): Efficacy and safety of levofloxacin preventive treatment in child and adolescent HHCs of multidrug-resistant tuberculosis (MDR-TB). Author: Anneke Hesseling⁷
V-QUIN MDR-TB prevention study: Levofloxacin versus placebo for the treatment of tuberculosis infection among contacts of patients with MDR-TB. Author: Greg Fox⁸
Methods
Annex 4. GRADE evidence-to-decision tables
Older terminology used in the context of TB preventive treatment (TPT), such as latent TB infection (LTBI) and active TB, has been retained in the original text of the tables.
PICO 1: What is the prevalence of TB infection, risk of progression to TB disease and cumulative prevalence of TB disease among household contacts without HIV in different age groups in high TB incidence countries?
Annex 3. GRADE summary of evidence tables
Older terminology used in the context of TPT, such as latent TB infection (LTBI) and active TB, has been retained in the original text of the tables.
PICO 1: What is the prevalence of TB infection, the risk of progression to TB disease and the cumulative prevalence of TB disease among household contacts without HIV in different age groups in high TB incidence countries?
Is the prevalence of TB disease and TB infection higher among household contacts without HIV than in the general population in different age groups in high TB incidence countries?
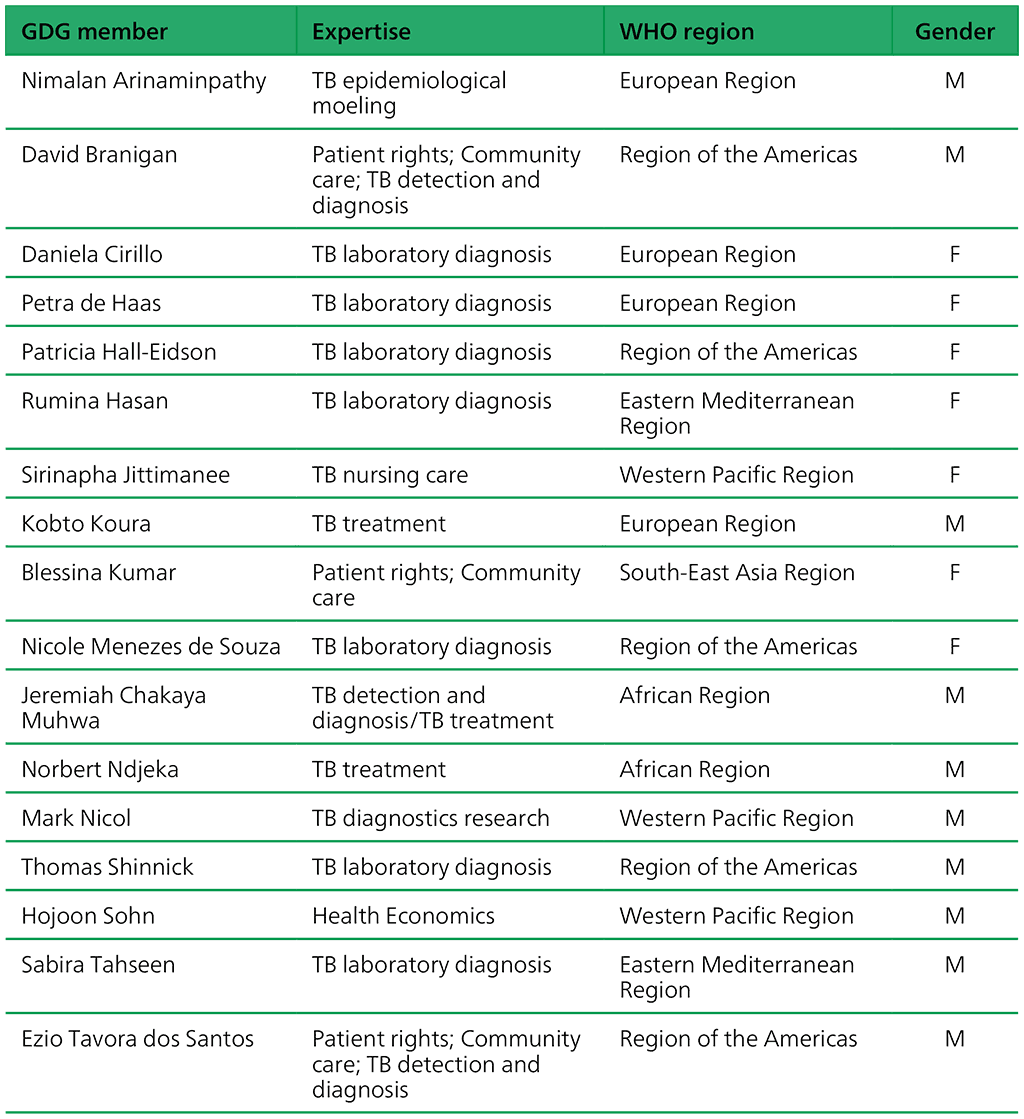
Annex 3: Guideline development group members
Table A.3.1. Guideline development group members: “Targeted next-generation sequencing” 2–5 May 2023

Pagination
- Page 1
- Next page
 Feedback
Feedback