Consolidated Guidelines
Abbreviations and acronyms
aDSM
active TB drug-safety monitoring and management
AFB
acid-fast bacilli
AIDS
acquired immunodeficiency syndrome
aIPD
adult individual patient data
1.6 Monitoring and evaluation
Patients who receive BPaLM/BPaL need to be tested at baseline and then monitored during treatment using schedules of relevant clinical and laboratory testing. If feasible, it is also important to follow up patients 12 months after the completion of treatment for possible relapse, including with sputum culture and smear.
References
- Global tuberculosis report 2019 (WHO/CDS/TB/2019.15). Geneva World Health Organization; 2019 (https://www.who.int/tb/publications/global_report/en/).
- Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis.
4.5 Monitoring and evaluation
Patients who receive the (H)REZ–levofloxacin regimen need to be monitored during treatment, using schedules of clinical and laboratory testing. The definitions to use when assigning outcomes are the same as those used for drug-susceptible TB (79). Signs of non-response or treatment failure should be followed up with DST for rifampicin resistance and, if possible, for fluoroquinolones and pyrazinamide.
4.4 Implementation considerations
Case scenarios
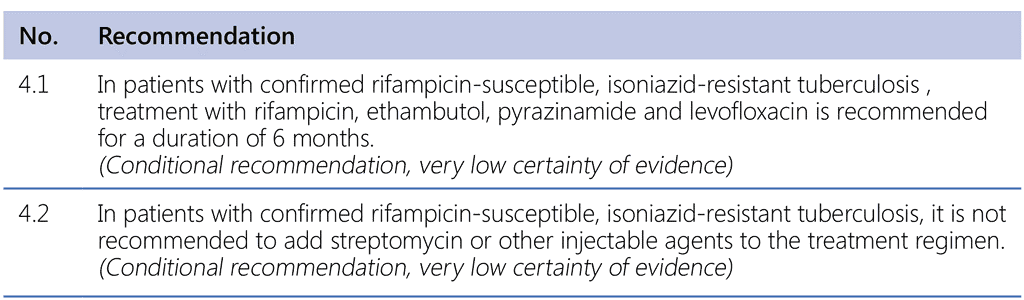
Implementing these recommendations requires the (H)REZ–levofloxacin regimen to be administered only in patients in whom resistance to isoniazid has been confirmed and resistance to rifampicin has been excluded. Preferably, testing for resistance to fluoroquinolones (and, if possible, to pyrazinamide) is also done before starting treatment. It is envisaged that the treatment regimen for Hr-TB will apply in the following situations:
4.3 Subgroup considerations
Children
In the IPD review, only 2% of Hr-TB patients were children; thus, a separate estimate of effect for paediatric patients was not possible. However, there is no reason why the results and recommendations cannot be extrapolated from adults to children, considering that the regimen components have been standard paediatric TB medicines for many years.
Patients with extensive disease
4.2 Justification and evidence
The recommendations in this section address one PICO question:
PICO question (Hr-TB, 2018): In patients with isoniazid-resistant TB (other than MDR-TB), which treatment regimen composition and duration, when compared with 6 months or more of rifampicin–pyrazinamide–ethambutol, leads to a higher likelihood of success with least possible risk of harm?
Pagination
- Previous page
- Page 2
- Next page
 Feedback
Feedback