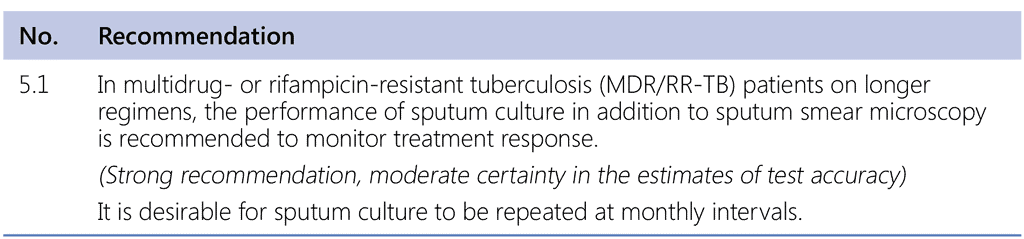
5.5 Monitoring and evaluation
Culture and microscopy results for tests performed in patients on MDR-TB treatment should be captured in the second-line TB treatment register as well as the respective laboratory registers (79). Sometimes these registers may exist as part of an electronic laboratory or patient information system, which makes it much easier for multiple users to access the data in real time and can also help to limit errors.
 Feedback
Feedback