4.2.9 Area 9 – Recording and reporting

Step 9.1 – Review and revise request for examination and reporting forms
 Feedback
Feedback

Step 9.1 – Review and revise request for examination and reporting forms

Step 8.1 – Implement a comprehensive quality assessment programme

Step 7.1 – Develop the use of digital data and diagnostics connectivity
TST and TBST results have the advantage of being provided by the clinical staff themselves. Nevertheless, the results should be adequately registered.

Step 6.1 – Develop SOPs
SOPs must be developed or adapted for TB infection skin or blood-based tests, for:
Uninterrupted availability at all testing sites of skin test materials, or reagents and disposables for IGRAs, is essential. Careful assessment of consumption rates is necessary to avoid shortage or excessive wastage of materials according to their shelf life.

Step 5.1 – Review forecasting, ordering and distribution procedures

Step 4.1 – Develop and implement a training curriculum and strategy

Step 3.1 – Select, procure, install and set up equipment
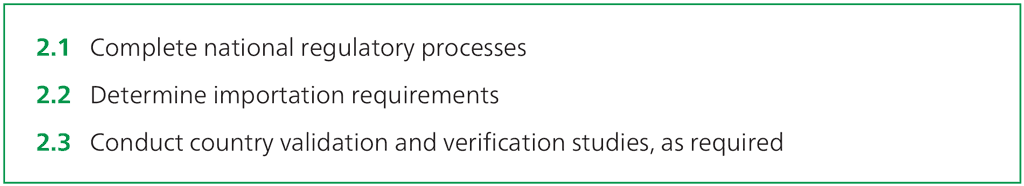
Step 2.1 – Complete national regulatory processes
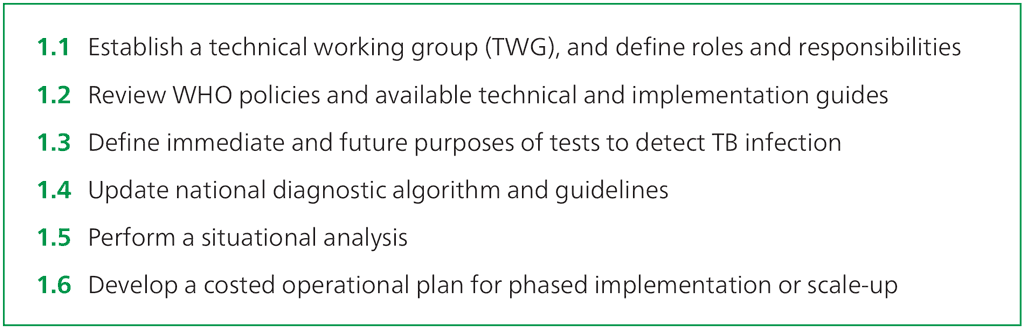
Step 1.1 – Establish a TWG, and define roles and responsibilities
The 10 main areas in implementing a new test are listed in Box 4.1. The following sections describe the steps within each of the 10 areas.
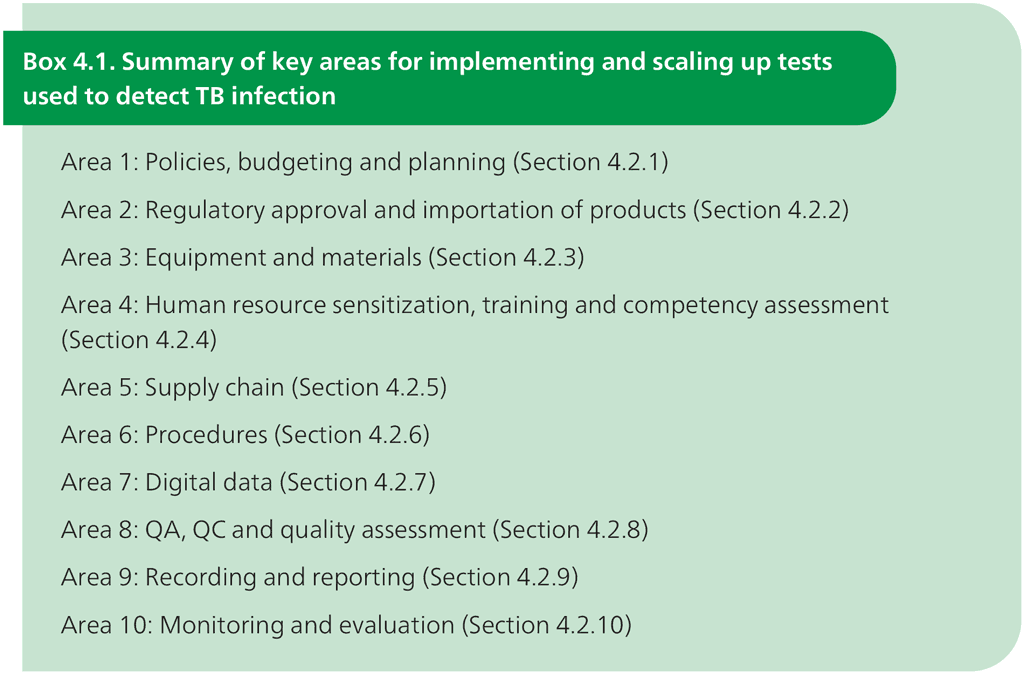
QA: quality assurance; QC: quality control; TB: tuberculosis.