Book traversal links for 4.4 Implementation considerations
Case scenarios
Implementing these recommendations requires the (H)REZ–levofloxacin regimen to be administered only in patients in whom resistance to isoniazid has been confirmed and resistance to rifampicin has been excluded. Preferably, testing for resistance to fluoroquinolones (and, if possible, to pyrazinamide) is also done before starting treatment. It is envisaged that the treatment regimen for Hr-TB will apply in the following situations:
- Hr-TB and rifampicin susceptibility are confirmed before TB treatment is started. Treatment with (H)REZ–levofloxacin is started immediately. If the diagnosis is strongly presumed (e.g. close contacts of a confirmed Hr-TB source case) but results of DST are still pending, the regimen may be introduced. If the DST results taken at the start eventually show susceptibility to isoniazid, then levofloxacin is stopped, and the patient continues treatment to complete a 2HREZ/4HR regimen.
- Hr-TB is confirmed after the start of treatment with the 2HREZ/4HR regimen. This includes patients who had undiagnosed isoniazid resistance initially or who developed isoniazid resistance later while on treatment with a first-line regimen. In such cases, rapid molecular testing for rifampicin resistance must be done (or repeated). Once rifampicin resistance has been excluded, a full 6-month course of (H)REZ–levofloxacin is given. The duration is driven by the need to give levofloxacin for 6 months, which usually implies that the companion first-line medicines are taken for longer than this.
If rifampicin resistance is detected, the patient needs to be started on a recommended MDR-TB treatment regimen, as described in other sections of these guidelines.
Diagnostic capabilities
The overall aim of TB treatment is to achieve cure without relapse in all patients, interrupting M. tuberculosis transmission and preventing the acquisition (or amplification) of additional drug resistance. Globally, Hr-TB is more prevalent than MDR-TB. Thus, all countries need to move towards universal testing of both isoniazid and rifampicin resistance at the start of TB treatment, and to ensuring careful selection of patients eligible for the (H)REZ–levofloxacin regimen.43 The minimum diagnostic capacity to appropriately implement these recommendations is rapid molecular testing for rifampicin resistance before the start of treatment with the Hr-TB regimen and, preferably, the ruling out of fluoroquinolone resistance using WHO-recommended tests.
Rapid molecular tests such as Xpert MTB/RIF, Xpert MTB/XDR and LPAs are preferred, to guide patient selection for the (H)REZ–levofloxacin regimen (92, 97).
Surveillance of DR-TB indicates that fluoroquinolone resistance among patients with rifampicin-susceptible TB is generally low worldwide (98). However, national data on the prevalence of fluoroquinolone resistance – including targeted or whole-genome sequencing to detect specific mutations associated with resistance to fluoroquinolones (52) – could help to guide testing policies when countries implement the Hr-TB treatment recommendations.
When additional resistance (e.g. to both fluoroquinolones and pyrazinamide) is suspected or confirmed, treatment regimens that include other second-line TB medicines may have to be designed individually. The current review could not provide further evidence on effective regimens in patients with polyresistant disease.
Support and close monitoring of patients are needed to maximize treatment adherence and enable early detection of patients who are not responding to treatment (e.g. those with persistent sputum culture or smear positivity). In the presence of non-response to treatment, DST for rifampicin and the fluoroquinolones should be repeated, preferably with Xpert MTB/XDR or LPA. Documented acquisition of resistance to rifampicin or a fluoroquinolone while on the Hr-TB treatment regimen should alert the clinician to the need to review the entire clinical and microbiological status of the patient, and change the regimen where necessary.
Levofloxacin is proposed as the fluoroquinolone of first choice in the Hr-TB treatment regimen for several reasons. First, the safety profile of this medicine is better characterized than that of other fluoroquinolones, and levofloxacin was the fluoroquinolone most frequently used in the studies reviewed for this guidance. Second, in comparison to moxifloxacin, levofloxacin has fewer known drug interactions with other medications. For example, although both plasma peak concentration and exposure to moxifloxacin decrease significantly when the drug is combined with rifampicin (99), the same effect has not been reported for levofloxacin, possibly because levofloxacin undergoes limited metabolism in humans and is excreted unchanged in the urine (100). Third, although levofloxacin may interfere with lamivudine clearance, in contrast to moxifloxacin, there are no contraindications for its use with other antiretroviral agents (36).
The addition of levofloxacin to (H)REZ is recommended in patients with Hr-TB, with the exception of the following situations:
- resistance to rifampicin cannot be excluded (i.e. unknown susceptibility to rifampicin, or indeterminate or error results on Xpert MTB/XDR);
- known or suspected resistance to levofloxacin;
- known intolerance to fluoroquinolones;
- known or suspected risk for prolonged QT interval; and44
- if possible, in pregnancy or during breastfeeding (not an absolute contraindication).
Sometimes, the confirmation of isoniazid resistance arrives late (e.g. 5 months into a 2HREZ/4HR regimen). In such cases, a decision to start 6 months of (H)REZ–levofloxacin depends on the patient’s clinical condition and microbiological status.
If levofloxacin cannot be used because of toxicity or resistance, the patient may be given 6(H)REZ as an alternative. Based on the results of the evidence review for these guidelines, replacement of levofloxacin with an injectable agent is NOT advised. The evidence review could not inform on the effect of other second-line TB medicines on treatment effectiveness.
Addition of isoniazid
There was no clear evidence that the addition of isoniazid affects patients (i.e. adding benefit or harm). For patient convenience and ease of administration, the four-drug HREZ FDCs45 may be used to deliver the Hr-TB treatment regimen alongside levofloxacin.
The use of high-dose isoniazid (10–15 mg/kg per day in adults) was not evaluated in this review owing to insufficient data. However, the GDG discussed the effect of increasing isoniazid dosing beyond that provided in weight-banded FDCs, depending on the type of molecular mutations identified. In vitro evidence suggests that when specific inhA mutations are detected (and when katG mutations are absent), increasing the dose of isoniazid is likely to be effective; thus, additional isoniazid up to a maximum dose of 15 mg/kg per day could be considered. In the case of katG mutations, which usually confer a higher level resistance, the use of isoniazid even at a higher dose is less likely to be effective (101).46
Dosage
Although the IPD analysis did not provide evidence to address the frequency of dosing, it is best to avoid intermittent or divided dosing of the 6(H)REZ–levofloxacin regimen (37, 102, 103). In the absence of full information about optimal drug doses, a weight-band dosing scheme for levofloxacin is recommended.47
Drug–drug interactions
Levofloxacin may interfere with lamivudine clearance (increasing the levels of lamivudine) but it is not contraindicated with other antiretroviral agents, and no drug dosing adjustments are needed (36). Co-administration of levofloxacin with oral divalent cation-containing compounds (e.g. antacids) may impair its absorption and should be avoided (10). Restriction of concomitant use of milk products is not necessary.
Treatment prolongation beyond 6 months
Prolonging of treatment beyond 6 months may be considered for patients with extensive disease or in those slow to convert to smear or culture negative. In the latter, acquisition of additional resistance to rifampicin must be ruled out, as must resistance to fluoroquinolones and pyrazinamide, if possible. Such patients require careful monitoring and follow-up.
Cost
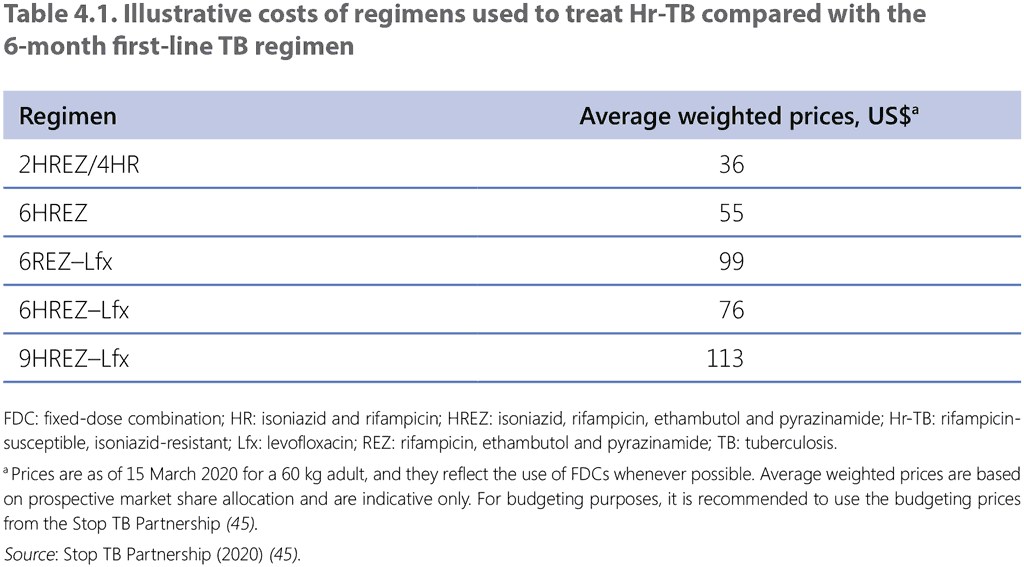
A cost–effectiveness analysis was not performed for this review. Table 4.1 presents approximate prices for a full course of medicines with the different regimens in adults, based on the cost of products available from the GDF (45). Use of FDCs, even for part of the regimen, reduces costs. Medicines needed for a 6HREZ–levofloxacin regimen cost about three times as much as a 2HREZ/4HR regimen when using the HREZ FDC. The treatment of Hr-TB according to these guidelines is not expected to significantly increase operational costs.
Adherence
The IPD analysis contained limited data on the treatment adherence strategies used, such as directly observed treatment and self-administered therapy (SAT). Improved treatment success rates appeared to be associated with increased patient support, including medication adherence support (e.g. by means of digital technologies) or other means, as recommended by WHO (37). In contrast to regimens for drug-susceptible TB and MDR-TB, the recommended Hr-TB treatment regimen does not have an intensive phase and a continuation phase, simplifying the delivery and monitoring of treatment.
43 The association between previous TB treatment history and Hr-TB is less strong than the association in MDR-TB. As a result, previous TB treatment is less reliable as a proxy for Hr-TB and a laboratory diagnosis is therefore important.
44 Baseline-corrected QT. Prolongation of the QT interval and isolated cases of torsades de pointes have been reported. Avoid use in patients with known prolongation, those with hypokalaemia, and with other drugs that prolong the QT interval.
45 Although most countries currently procure the four-drug FDC via the Stop TB Partnership’s GDF, in settings where only the three-drug combination FDC (i.e. HRZ) is available, ethambutol has to be administered separately.
46 An isolated katG or inhA mutation can correspond to variable MIC levels. This implies that inhA mutations do not always indicate low-level isoniazid resistance, and that katG mutations are not necessarily correlated with high-level isoniazid resistance. However, the presence of both mutations is usually an indication of high-level resistance (101).
47 Studies included in this IPD analysis involved the use of regimens containing levofloxacin (usually at a dose of 750–1 000 mg/day), moxifloxacin (400 mg/day) or gatifloxacin (400 mg/day), as well as early-generation fluoroquinolones (ciprofloxacin and ofloxacin), which are no longer recommended for the treatment of DR-TB. Gatifloxacin is currently unavailable in quality-assured formulations, and ciprofloxacin and ofloxacin are no longer recommended for use in DR-TB care.
 Feedback
Feedback