1.5 Implementation considerations
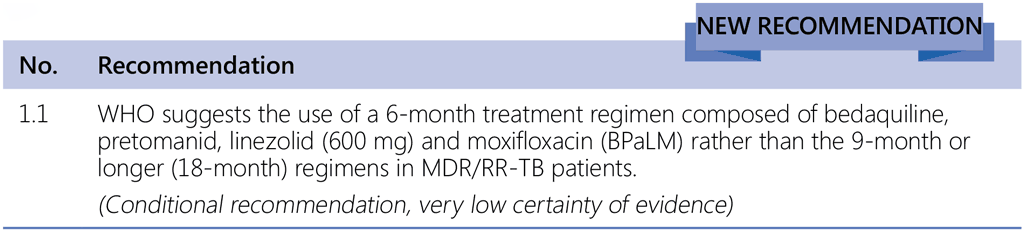
High treatment success rates shown for the BPaLM and BPaL regimens in the Nix-TB study and in the ZeNix and TB-PRACTECAL trials, and favourable comparison with the current SoC regimens led to thorough discussions during the GDG meeting of an overall recommendation for implementation under routine programmatic conditions and of the implementation considerations for this regimen.
 Feedback
Feedback