Book traversal links for Executive summary
Tuberculosis (TB) strains that are resistant to TB medicines are more difficult to treat than drug-susceptible ones, and present a major challenge for patients, health care workers and health care services. In addition, the increase of drug-resistant TB (DR-TB) threatens global progress towards the targets set by the End TB Strategy4 of the World Health Organization (WHO). Thus, there is a critical need for the continual development of evidence-based policy recommendations on the treatment and care of patients with DR-TB, based on the most recent and comprehensive evidence available.
In the past decade, WHO has developed and issued evidence-based policy recommendations for the treatment and care of patients with DR-TB, published in a range of documents (see Box 1). WHO has recently started to consolidate guidelines, in response to requests from Member States, to facilitate policy transfer at the country level. The first integrated recommendations for the management and care of multidrug- or rifampicin-resistant TB (MDR/RR-TB) were released in 2019, as the WHO consolidated guidelines on drug-resistant tuberculosis treatment.5 The consolidation of WHO recommendations on TB and DR-TB has now been expanded to better outline the path that a patient will take following exposure to resistant strains of Mycobacterium tuberculosis, once infection has progressed to TB disease, and the patient has been identified by the health system and referred for DR-TB treatment.
The guidance provided in this sub-module under TB treatment policy outlines specific WHO recommendations on the overall treatment management, care and monitoring of patients with MDR/RR-TB. It brings forward recommendations developed by various Guideline Development Groups (GDGs) convened by WHO. The GDGs use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to summarize the evidence, and formulate policy recommendations and accompanying remarks (Sections 2–7). This sub-module also incorporates new recommendations that were made in February and March 2022 (Sections 1–2), based on new evidence that was available to WHO on the following: the use of the bedaquiline, pretomanid,6 linezolid and moxifloxacin (BPaLM) regimen for patients with MDR/RR-TB, and the use of 9-month all-oral bedaquiline-containing regimens for patients with MDR/RR-TB. The inclusion of these two new recommendations in the current update of the consolidated guidelines was communicated to the public via a rapid communication in May 2022.7 This rapid communication was released in advance of updated WHO consolidated guidelines, to inform national TB programmes (NTPs) and other stakeholders of key changes in the treatment of DR-TB and to allow for rapid transition and planning at the country level.
Overall, this sub-module focuses on recommendations for the use of effective treatment regimens for people with DR-TB; specifically, regimens for rifampicin-susceptible, isoniazid-resistant TB (Hr-TB), all-oral shorter regimens for MDR/RR-TB, longer regimens for MDR/RR-TB, monitoring the patient response to MDR/RR-TB treatment, starting antiretroviral therapy (ART) in patients on second-line anti-TB regimens and providing surgery for patients on MDR-TB treatment. Additionally, to inform the global community of the major gaps and research areas to be addressed and to inform the development of evidence-based recommendations, this document outlines the research priorities that will help to generate knowledge on evidence-based and attainable standards of health.
In this updated document, stakeholders will be able to distinguish between previous recommendations that remain valid, previous recommendations that have been updated, and new recommendations that have been developed based on additional studies, considering the range of known benefits and potential harms, modelling exercises and other data to inform the decision-making process.
The recommendations included herein are a component of the WHO consolidated guidelines on TB and are primarily intended for use by NTPs, public health agencies, and other key constituencies involved in the planning, implementation and monitoring of activities for the programmatic management of DR-TB.
The methods used to develop and formulate the recommendations complied with WHO standards for guideline development, and were based on up-to-date evidence reviews, complemented with additional information on values and preferences, feasibility and acceptability, and cost. The GRADE approach was used to rate the certainty in the estimate of effect (i.e. quality of evidence) as high, moderate, low or very low; it was also used to determine the strength of the recommendations, rating them as strong or conditional.
Current WHO recommendations on the treatment of DR-TB
The recommendations for the treatment of DR-TB that are presented in this document have been derived from earlier WHO guideline documents (Box 1), and a WHO guideline development conducted in February–March 2022. These recommendations supersede the WHO consolidated guidelines on tuberculosis. Module 4: Treatment – drug-resistant tuberculosis treatment, that were published in 2020.8
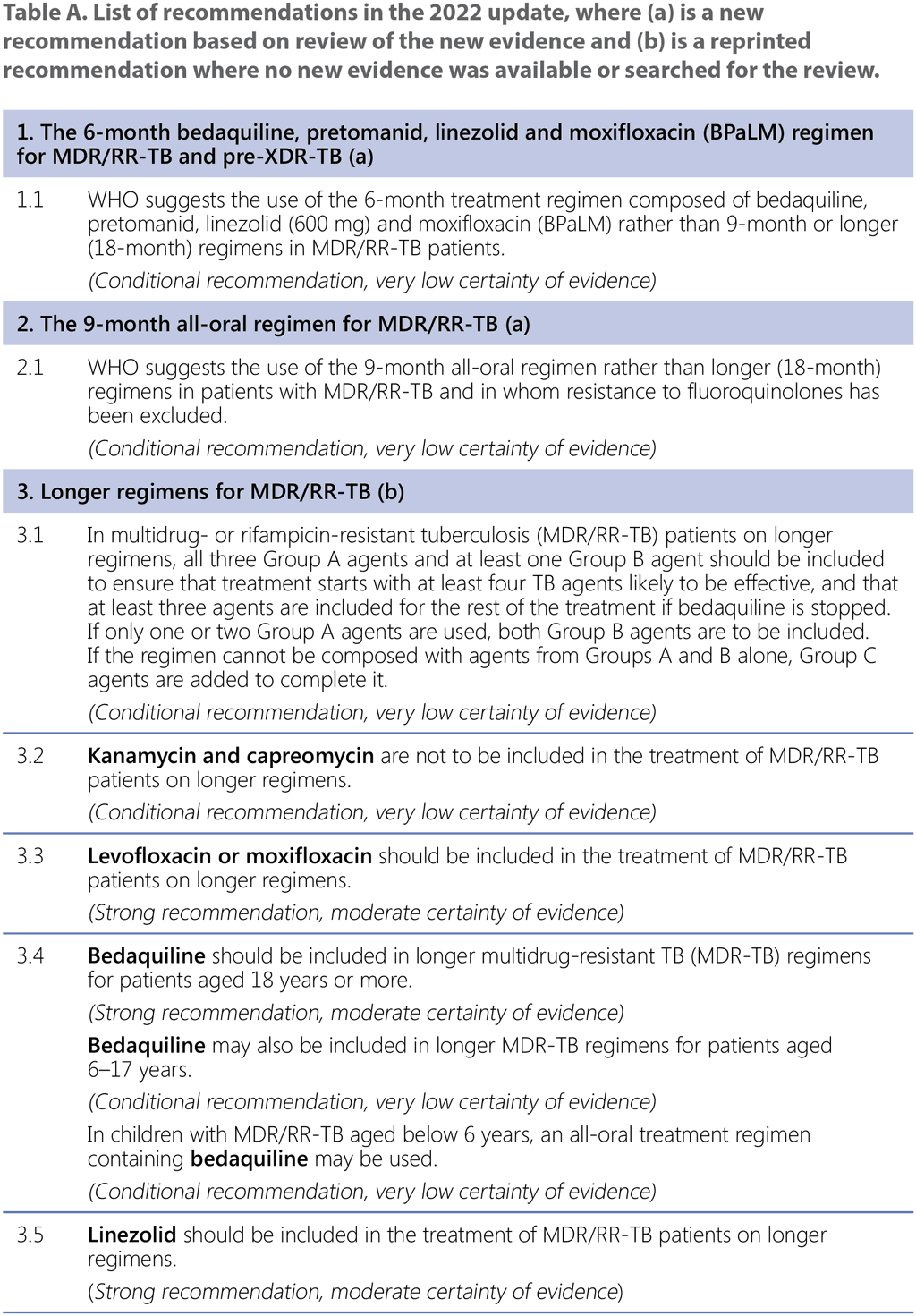
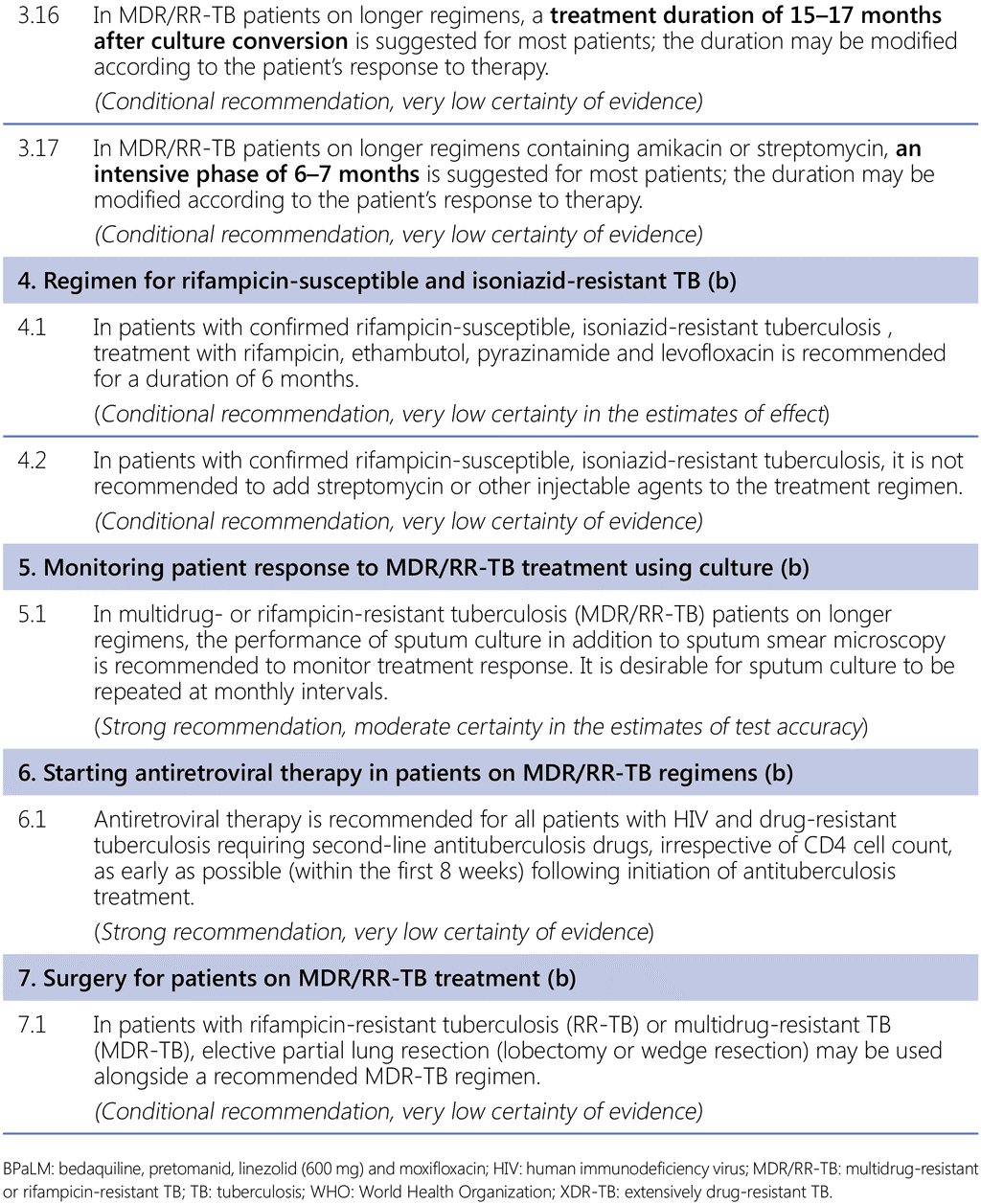
This module contains recommendations on treatment regimens for MDR/RR-TB and Hr-TB, including all-oral shorter and longer regimens for MDR/RR-TB, monitoring of patients on treatment, the timing of ART in MDR/RR-TB patients living with HIV and the use of surgery for patients receiving MDR-TB treatment. The recommendations are presented in Table A below and labelled as either a new recommendation (where based on a review of new evidence) or a reprinted recommendation (where no new evidence was available or searched for the review).

4 The End TB Strategy. Geneva: World Health Organization; 2015 (https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy).
5 WHO consolidated guidelines on drug-resistant tuberculosis treatment (WHO/CDS/TB/2019.7). Geneva: World Health Organization; 2019 (https://apps.who.int/iris/handle/10665/311389).
6 Pretomanid is a new chemical entity and a member of a class of compounds known as nitroimidazo-oxazines, which possess significant anti-TB activity and a unique mechanism of action.
7 Rapid communication: key changes to the treatment of drug-resistant tuberculosis. Geneva: World Health Organization; 2022 (https://www.who.int/publications/i/item/WHO-UCN-TB-2022–2).
8 WHO consolidated guidelines on tuberculosis. Module 4: Treatment – drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2020 (https://www.who.int/publications/i/item/9789240007048).
9 Imipenem–cilastatin and meropenem are administered with clavulanic acid, which is available only in formulations combined with amoxicillin. Amoxicillin–clavulanic acid is not counted as an additional effective TB agent, and it should not be used without imipenem–cilastatin or meropenem.
 Feedback
Feedback