Book traversal links for 1.3 Evidence to recommendations: considerations
Based on the decisions taken during the review and the combination of assessments described above, the new recommendation is to use the BPaLM regimen as the first choice in the defined patient group with MDR/RR-TB, with the regimen to be used under routine programmatic conditions. Patients with MDR/RR-TB who are not eligible for this regimen can be treated using one of the 9-month regimens (see Section 2). The use of the longer regimen is reserved (see Section 3) for individuals with MDR/RR-TB and fluoroquinolone resistance with further resistance or intolerance to bedaquiline, linezolid (XDR-TB) or pretomanid, who would then receive a longer regimen designed with remaining effective medicines from Groups A, B and C, according to their drug susceptibility profile and other parameters.
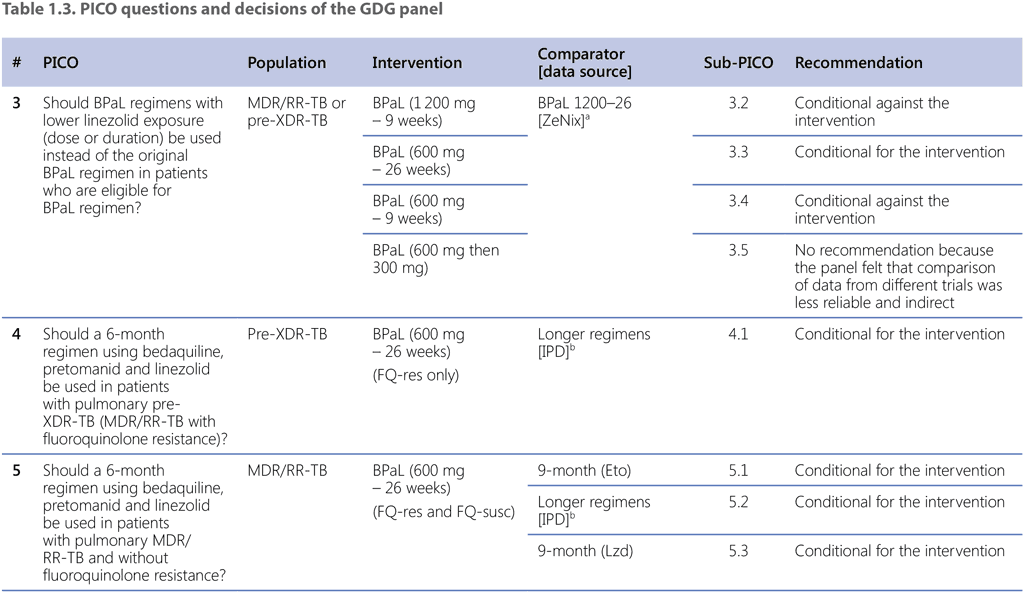
Table 1.3 lists the comparisons and decisions on each of the sub-PICO-questions that were eventually used by the GDG to conclude with this summary recommendation. Throughout the discussion, the GDG panel focused on direct (within trial) comparisons among the TB-PRACTECAL trial arms, to ensure consistency and because it was felt that results based on random allocation to interventions were far more reliable than indirect, nonrandomized comparisons. Whereas the certainty of evidence of these (TB-PRACTECAL-internal) comparisons was still judged to be very low, the panel deemed it to be higher than that of other (indirect or between-trial or cohort) comparisons.
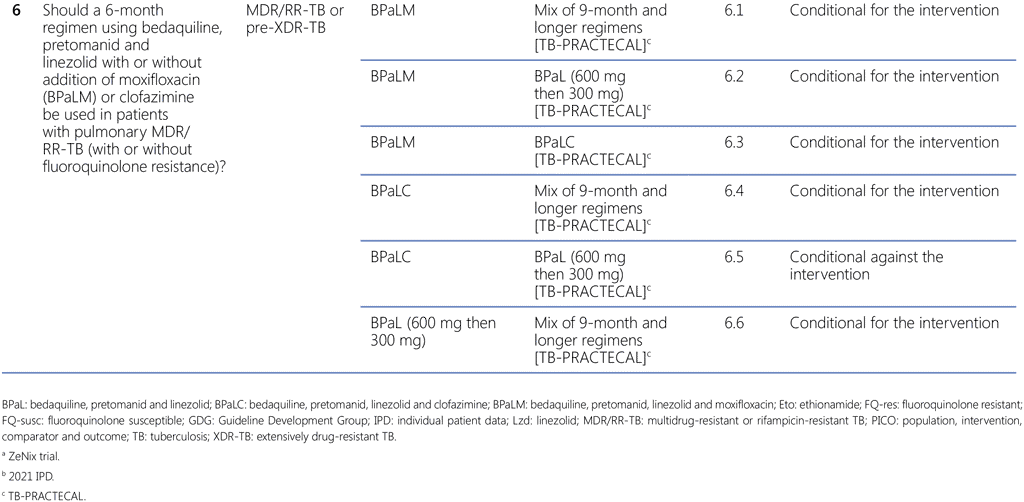
Although assessments of PICO questions 3, 4, 5 and 6 have all contributed to the summary recommendation, the main assessment that defined the overall decision was that of sub-PICO 6.1 on the comparison of the BPaLM regimen of the stage 2 (corresponds to Phase 3) in the TB-PRACTECAL trial with the mix of SoC regimens (conforming to the WHO-recommended 9-month or longer regimens). Even though the TB-PRACTECAL trial was not designed to compare the investigational regimens against each other and with the SoC, the comparisons of the different arms of the trial with the BPaLM arm (sub-PICOs 6.2–6.6) were performed to aid the panel in making final decisions.
The assessment of PICO 3 allowed for the decision on the optimal dosing and duration of linezolid within the BPaLM/BPaL regimen and narrowed down the subsequent comparisons to the intervention regimen with this particular dose and duration of linezolid – BPaL (600 mg – 26 weeks). The justification for how the other assessments have contributed to the overall recommendation can be summarized as follows:
- The assessment of PICO 4 resulted in the conditional recommendation for use of BPaL (600 mg – 26 weeks) regimen over the currently recommended longer regimens in patients with MDR/RR-TB and additional fluoroquinolone resistance.
- The three assessments performed under PICO 5 resulted in the conditional recommendations for the BPaL (600 mg – 26 weeks) regimen over the currently recommended 9-month regimen with ethionamide (sub-PICO 5.1), over longer regimens (sub-PICO 5.2) and over the new 9-month regimen where ethionamide is replaced with 2 months of linezolid (sub-PICO 5.3) in patients with pulmonary MDR/RR-TB without fluoroquinolone resistance.
- The assessment of sub-PICO 6.1 resulted in the conditional recommendation for use of the BPaLM regimen of the TB-PRACTECAL trial over the comparator, the mix of SoC regimens under this trial conforming to the WHO recommendations on 9-month or longer regimens, depending on the trial site.
- The assessments of sub-PICOs 6.4 and 6.6 resulted in the conditional recommendations for BPaLC and BPaL over the SoC in the TB-PRACTECAL trial; thus all three 6-month BPaL-based regimens were assessed to be preferred over the mix of SoC regimens under this trial.
- The assessments of sub-PICOs 6.3 and 6.5 resulted in the conditional recommendations for BPaLM and BPaL over BPaLC; based on these assessments the GDG concluded that BPaLC should not be recommended as a regimen.
- The assessment of sub-PICO 6.2 resulted in the conditional recommendations for BPaLM over BPaL; thus, it highlighted the use of the BPaLM regimen as the preferred regimen under the conditions specified in the recommendation and remarks. Compared with BPaL, BPaLM led to more treatment success, fewer failures or recurrences and less emerging drug resistance while showing little difference in adverse events.
The GDG panel discussed the subgroups and implementation considerations, and the monitoring and evaluation and research priorities as they pertain to the summary recommendation rather than for each individual sub-PICO question.
 Feedback
Feedback