Book traversal links for Xpert MTB/RIF and Xpert MTB/RIF Ultra assays
The development of the Xpert MTB/RIF assay (Cepheid, Sunnyvale, United States of America [USA]) was a significant step forward for improving the diagnosis of TB and the detection of rifampicin resistance globally. However, Xpert MTB/RIF sensitivity is suboptimal, particularly in smear-negative and HIV-associated TB patients. The Xpert MTB/RIF Ultra (Cepheid, Sunnyvale, USA), hereafter referred to as Xpert Ultra, was developed by Cepheid as the next-generation assay to overcome these limitations. It uses the same GeneXpert® platform as the Xpert MTB/RIF.
Recommendations
This section contains five sets of recommendations, with each set being specific for a particular type of testing (initial or repeated) and type of TB (pulmonary or extrapulmonary).
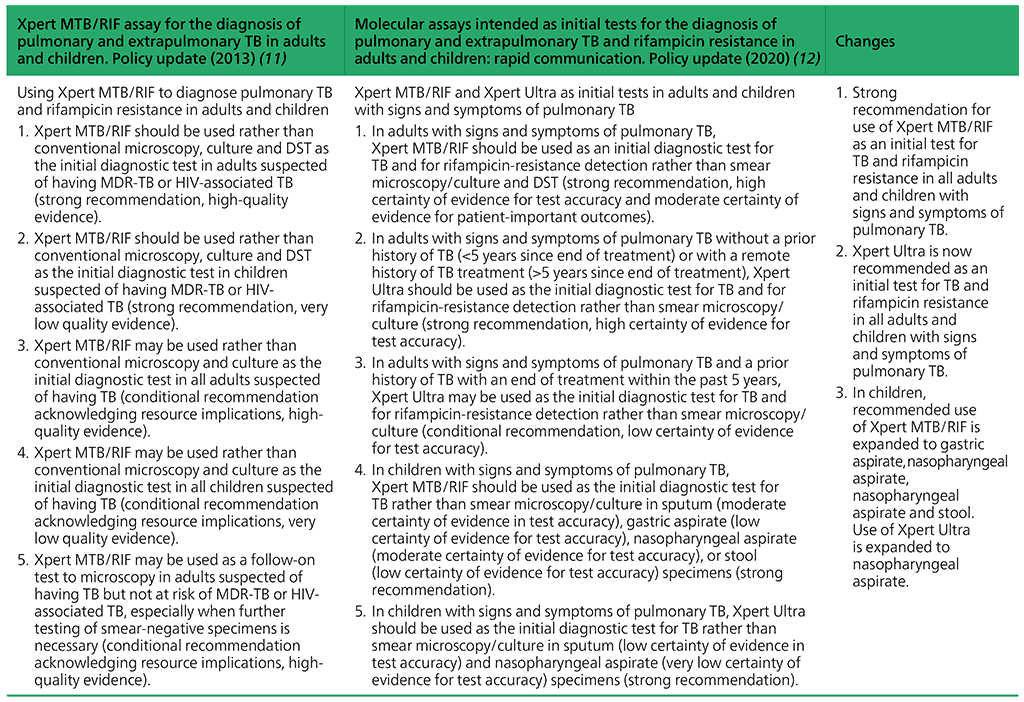
Recommendations on Xpert MTB/RIF and Xpert Ultra as initial tests in adults and children with signs and symptoms of pulmonary TB
- In adults with signs and symptoms of pulmonary TB, Xpert MTB/RIF should be used as an initial diagnostic test for TB and rifampicin-resistance detection in sputum rather than smear microscopy/culture and phenotypic DST.
(Strong recommendation, high certainty of evidence for test accuracy; moderate certainty of evidence for patient-important outcomes⁶) - In children with signs and symptoms of pulmonary TB, Xpert MTB/RIF should be used as an initial diagnostic test for TB and rifampicin-resistance detection in sputum, gastric aspirate, nasopharyngeal aspirate and stool rather than smear microscopy/ culture and phenotypic DST.
(Strong recommendation, moderate certainty for accuracy in sputum; low certainty of evidence for test accuracy in gastric aspirate, nasopharyngeal aspirate and stool) - In adults with signs and symptoms of pulmonary TB and without a prior history of TB (≤5 years) or with a remote history of TB treatment (>5 years since end of treatment), Xpert Ultra should be used as an initial diagnostic test for TB and for rifampicin-resistance detection in sputum, rather than smear microscopy/culture and phenotypic DST.
(Strong recommendation, high certainty of evidence for test accuracy) - In adults with signs and symptoms of pulmonary TB and with a prior history of TB and an end of treatment within the last 5 years, Xpert Ultra may be used as an initial diagnostic test for TB and for rifampicin-resistance detection in sputum, rather than smear microscopy/culture and phenotypic DST.
(Conditional recommendation, low certainty of evidence for test accuracy) - In children with signs and symptoms of pulmonary TB, Xpert Ultra should be used as the initial diagnostic test for TB and detection of rifampicin resistance in sputum or nasopharyngeal aspirate, rather than smear microscopy/culture and phenotypic DST.
(Strong recommendation, low certainty of evidence for test accuracy in sputum; very low certainty of evidence for test accuracy in nasopharyngeal aspirate)
Remarks
For recommendation 2: Sputum includes expectorated and induced sputum. Studies assessing the impact of Xpert MTB/RIF on outcomes in children are lacking. The choice of the specimen will depend on the acceptability (for children, parents, health care workers and other stakeholders) and the feasibility of collecting and preparing specimens in the local context. Regarding Xpert MTB/RIF, the certainty of evidence is higher for sputum and nasopharyngeal aspirates than for other specimen types. The recommendation can be extrapolated for children living with HIV. The direct benefit from testing for rifampicin resistance in sputum (very low certainty of evidence for test accuracy) can be extrapolated to other specimens.
For recommendation 4: The justification for a conditional recommendation is based on:
- low certainty of evidence for test accuracy;
- uncertainty about the interpretation of Xpert Ultra trace results in patients with a prior history of disease and the associated high false-positivity rate; and
- uncertainty about the required resources.
For patients with Xpert Ultra trace results, decisions regarding treatment initiation should include considerations of the clinical presentation and the patient context (including prior treatment history, probability of relapse and other test results).
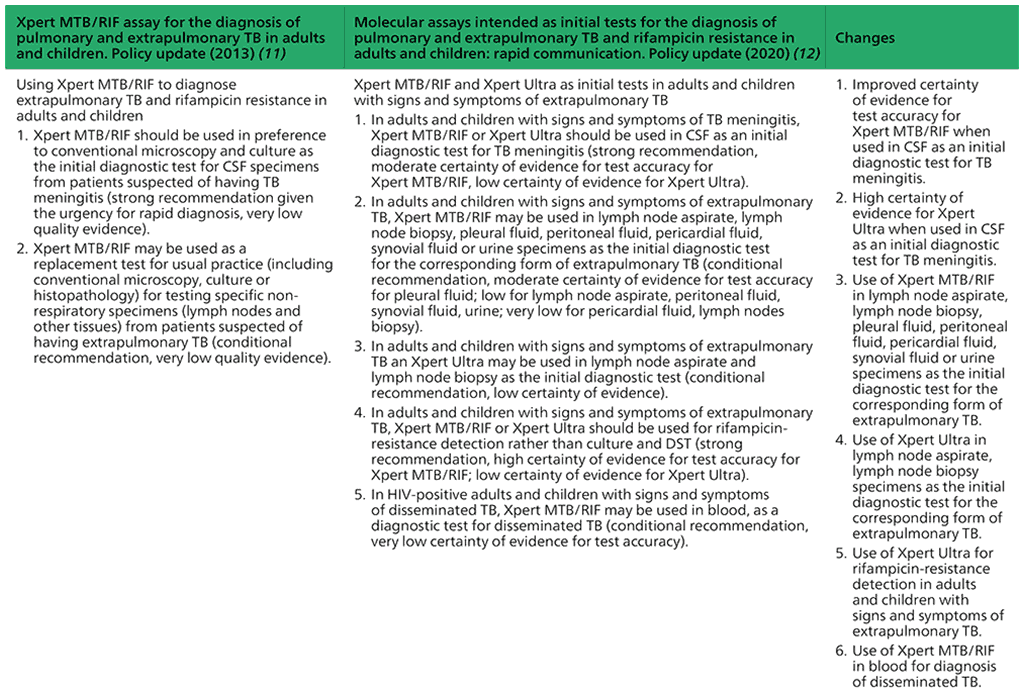
Recommendations on Xpert MTB/RIF and Xpert Ultra as initial tests in adults and children with signs and symptoms of extrapulmonary TB
- In adults and children with signs and symptoms of TB meningitis, Xpert MTB/RIF or Xpert Ultra should be used in cerebrospinal fluid (CSF) as an initial diagnostic test for TB meningitis rather than smear microscopy/culture.
(Strong recommendation, moderate certainty of evidence for test accuracy for Xpert MTB/RIF; low certainty of evidence for test accuracy for Xpert Ultra) - In adults and children with signs and symptoms of extrapulmonary TB, Xpert MTB/ RIF may be used in lymph node aspirate, lymph node biopsy, pleural fluid, peritoneal fluid, pericardial fluid, synovial fluid or urine specimens as the initial diagnostic test rather than smear microscopy/culture.
(Conditional recommendation, moderate certainty of evidence for test accuracy for pleural fluid; low certainty for lymph node aspirate, peritoneal fluid, synovial fluid, urine; very low certainty for pericardial fluid, lymph nodes biopsy) - In adults and children with signs and symptoms of extrapulmonary TB, Xpert Ultra may be used in lymph node aspirate and lymph node biopsy as the initial diagnostic test rather than smear microscopy/culture.
(Conditional recommendation, low certainty of evidence) - In adults and children with signs and symptoms of extrapulmonary TB, Xpert MTB/ RIF or Xpert Ultra should be used for rifampicin-resistance detection rather than culture and phenotypic DST.
(Strong recommendation, high certainty of evidence for test accuracy for Xpert MTB/RIF; low certainty of evidence for Xpert Ultra) - In HIV-positive adults and children with signs and symptoms of disseminated TB, Xpert MTB/RIF may be used in blood, as an initial diagnostic test for disseminated TB.
(Conditional recommendation, very low certainty of evidence for test accuracy)
Remarks
For recommendation 6: This recommendation applies to all patients with signs and symptoms of TB meningitis. The recommendation in children with signs and symptoms of TB meningitis is based on very low certainty of evidence for test accuracy for Xpert MTB/RIF. No data were available on the accuracy of Xpert Ultra for TB meningitis in children.
For recommendation 7: Clinical judgement and pretest probability should guide treatment. In a high pretest probability setting (>5%), a negative test result will not rule out the condition. Available data on Xpert MTB/RIF for children have included lymph node aspirate and lymph node biopsy specimens; given the similarity of the effects, the recommendation for adults is extrapolated for children.
For recommendation 8: The composite reference standard for Xpert Ultra gave similar results when lymph nodes aspirate was compared to lymph nodes biopsy.
For recommendation 9: Clinical judgement and pretest probability should guide treatment. In a high pretest probability setting, a negative test result will not rule out the condition.
For recommendation 10: Blood was only evaluated in people living with HIV (PLHIV) and under particular processing specifications (6), using third-generation Xpert MTB/RIF cartridges, based on one study with a small number of participants. The recommendation applies only to a particular population (HIV-positive adults with signs and symptoms of disseminated TB). The GDG did not feel comfortable extrapolating this recommendation to other patient populations.
Recommendations on Xpert MTB/RIF and Xpert Ultra repeated testing in adults and children with signs and symptoms of pulmonary TB ⁷
- In adults with signs and symptoms of pulmonary TB who have an Xpert Ultra trace positive result on the initial test, repeated testing with Xpert Ultra may not be used.
(Conditional recommendation, very low certainty of evidence for test accuracy) - In children with signs and symptoms of pulmonary TB in settings with pretest probability below 5% and an Xpert MTB/RIF negative result on the initial test, repeated testing with Xpert MTB/RIF in sputum, gastric fluid, nasopharyngeal aspirate or stool specimens may not be used.8
(Conditit onal recommendation, low certainty of evidence for test accuracy for sputum and very low for other specimen types) - In children with signs and symptoms of pulmonary TB in settings with pretest probability 5% or more and an Xpert MTB/RIF negative result on the initial test, repeated testing with Xpert MTB/RIF (for total of two tests) in sputum, gastric fluid, nasopharyngeal aspirate and stool specimens may be used.
(Conditional recommendation, low certainty of evidence for test accuracy for sputum and very low for other specimen types) - In children with signs and symptoms of pulmonary TB in settings with pretest probability below 5% and an Xpert Ultra negative result on the initial test, repeated testing with Xpert Ultra in sputum or nasopharyngeal aspirate specimens may not be used.
(Conditional recommendation, very low certainty of evidence for test accuracy) - In children with signs and symptoms of pulmonary TB in settings with pretest probability 5% or more and an Xpert Ultra negative result on the first initial test, repeated one Xpert Ultra test (for a total of two tests) in sputum and nasopharyngeal aspirate specimens may be used.
(Conditional recommendation, very low certainty of evidence for test accuracy)
Remarks
For recommendation 11: Xpert Ultra trace results will require follow-up, including reassessing clinical symptoms and information on prior history of TB. In the case of suspected rifampicin resistance, repeated testing may provide additional benefit for detection as well as an initial attempt to assess rifampicin resistance.
For recommendation 13: The GDG felt that the implementation of the recommendation depends on the acceptability (for children, parents or caregivers, health care workers and other stakeholders) and the feasibility of conducting repeated testing in the local context. The evidence reviewed evaluated repeating the same test on the same type of specimen. However, from the data reviewed on comparing single tests on different specimen types, there appears to be no difference, regardless of which second specimen is obtained. The recommendation can be extrapolated for children living with HIV (for Xpert MTB/RIF). This includes consideration of the direct benefit from detecting rifampicin resistance in sputum samples (very low certainty of evidence for test accuracy), which the GDG felt can be extrapolated to other samples. The recommendation applies to a moderate or high pretest setting (>5%). If the first test result is positive, the test should not be repeated. In settings with moderate to high pretest probability, the incremental yield of more than two tests is unknown.
For recommendation 15: Desirable and undesirable effects were judged to be moderate, but the GDG felt that testing twice in the moderate and high pretest probability (>5%) settings on balance may provide more benefits than harms. The recommendation is applicable for sputum and nasopharyngeal aspirates. No evidence was identified for stool and gastric aspirates.
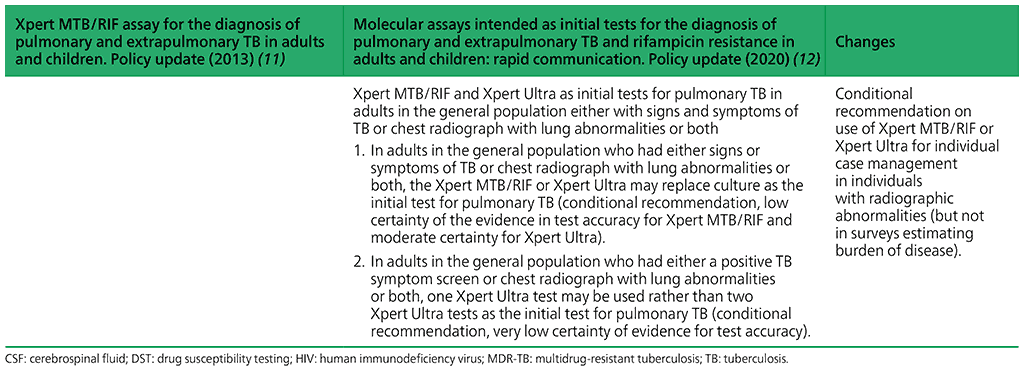
Recommendations on Xpert MTB/RIF and Xpert Ultra as initial tests for pulmonary TB in adults in the general population either with signs and symptoms of TB or chest radiograph with lung abnormalities or both ⁹
- In adults in the general population who had either signs or symptoms of TB or chest radiograph with lung abnormalities or both, the Xpert MTB/RIF or Xpert Ultra may replace culture as the initial test for pulmonary TB.
(Conditional recommendation, low certainty of the evidence in test accuracy for Xpert MTB/RIF and moderate certainty for Xpert Ultra) - In adults in the general population who had either a positive TB symptom screen or chest radiograph with lung abnormalities or both, one Xpert Ultra test may be used rather than two Xpert Ultra tests as the initial test for pulmonary TB.
(Conditional recommendation, very low certainty of evidence for test accuracy)
Remarks
For recommendation 16: This recommendation was informed by evidence from recent national surveys of TB disease prevalence in four high TB burden countries. Indirectness of the evidence was classified as serious, given that the methods applied in TB prevalence surveys differ from usual programmatic conditions (e.g. symptom screen limited to cough for 14 days or more, and a requirement in surveys to have the results of both symptom screen and chest radiography available). In addition, inconsistency of the evidence was also classified as serious, owing to variability of the data from different countries. As a result, certainty in the estimates of effect was downgraded to low for sensitivity and moderate for specificity. The recommendation applies only to the use of Xpert MTB/RIF or Xpert Ultra for clinical case management in situations where an immediate decision on patient treatment needs to be made and recourse to supplementary tests is not available or would incur delays. It does not apply to scientific studies with other objectives, such as the reliable estimation of the prevalence of TB disease in the community, for which alternative testing algorithms are required (in particular, to address the issue of false positive results, as illustrated in Table 1.17). Recommendations about the screening and diagnostic algorithms to be used in such studies are beyond the scope of this GDG. Recommendations for the diagnostic algorithm(s) to recommend in national TB prevalence surveys specifically are being developed by WHO and are scheduled for release in 2020.
For recommendation 17: There are concerns about losing global and national capacity for culture testing – the current reference standard for identifying active TB disease. An Xpert Ultra trace result was considered as negative in these studies. More false positive results are expected for Xpert Ultra for pulmonary TB. The recommendation applies only to the use of Xpert Ultra for clinical case management. When Xpert Ultra gives a positive result, clinical management should be followed according to national guidelines. When Xpert Ultra gives a negative result, the patient should be re-evaluated clinically. In the case of a culture-positive result, clinical management should be followed according to national guidelines. In the case of a culture-negative result, the patient should be re-evaluated clinically. The recommendation does not apply to scientific studies with other objectives, such as the reliable estimation of the prevalence of TB disease in the community, in which alternative testing algorithms (e.g. using more than one test) may be required. Recommendations for the diagnostic algorithm(s) to be used in such studies are beyond the scope of this GDG. Recommendations for the diagnostic algorithm(s) to recommend in national TB prevalence surveys specifically are being developed by WHO and are scheduled for release in 2020.
Test descriptions
Xpert MTB/RIF is an automated PCR test (molecular test) using the GeneXpert platform (Fig. 2.1.1). Xpert MTB/RIF is a single test that can detect both MTBC bacteria and rifampicin resistance within 2 hours of starting the test, with minimal hands-on technical time (7).
Fig. 2.1.1. The GeneXpert four-module instrument and the Xpert MTB/RIF test cartridge

In Xpert MTB/RIF sample processing – in contrast to conventional nucleic acid amplification tests (NAATs) – PCR amplification and detection are integrated into a single self-enclosed test unit; that is, the Xpert MTB/RIF cartridge. Following sample loading, all steps in the assay are automated and contained within the cartridge. In addition, the assay’s sample reagent, used to liquefy sputum, is tuberculocidal (i.e. it has the ability to kill TB bacteria), which largely eliminates concerns about biosafety during the test procedure. These features allow the technology to be taken out of a central laboratory or reference laboratory, and be used nearer to patients. However, Xpert MTB/RIF requires an uninterrupted and stable electrical power supply, temperature control and yearly calibration of the instrument’s modules (8).
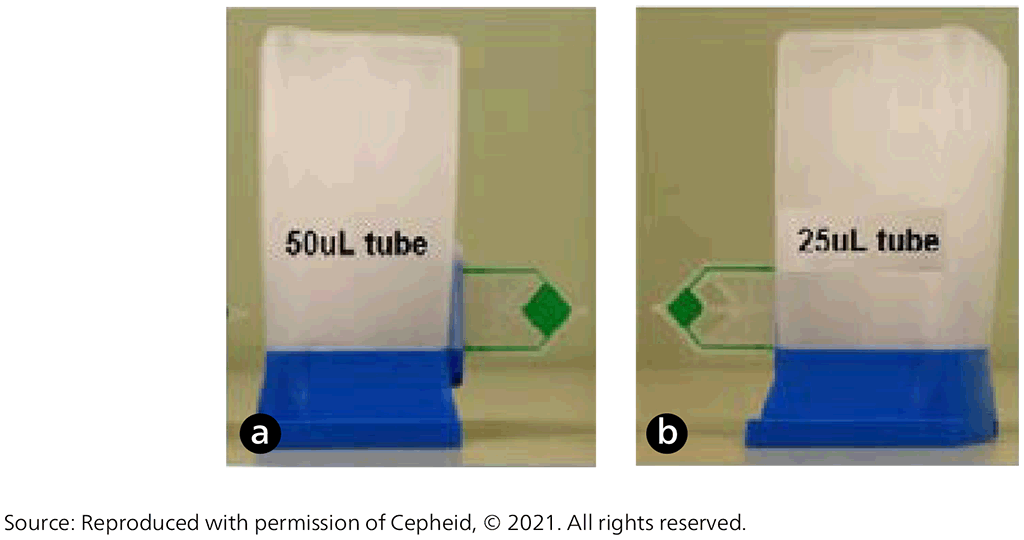
Xpert Ultra uses the same GeneXpert platform as Xpert MTB/RIF; Cepheid developed it as the next-generation assay to overcome limitations in sensitivity for TB diagnosis. To improve assay sensitivity for the detection of MTBC, the Xpert Ultra assay incorporates two different multicopy amplification targets (IS6110 and IS1081) and has a larger DNA reaction chamber than Xpert MTB/RIF (50 µL PCR in Xpert Ultra versus 25 µL in Xpert MTB/RIF, Fig. 2.1.2). Xpert Ultra also incorporates fully nested nucleic acid amplification, more rapid thermal cycling, and improved fluidics and enzymes. This has resulted in Xpert Ultra having a limit of detection of 16 bacterial colony forming units (cfu) per millilitre (compared with 114 cfu/mL for Xpert MTB/RIF). To improve the accuracy of rifampicin-resistance detection, the Xpert Ultra test incorporates melting-temperature-based analysis. Specifically, four probes identify rifampicin-resistance mutations in the rifampicin-resistance determining region of the rpoB gene by detecting shifts in the melting temperature away from the wild-type reference range (9).
Fig. 2.1.2. (a) The Xpert MTB/RIF Ultra cartridge with its 50 µL reaction tube (green) and (b) the Xpert MTB/RIF cartridge with its 25 µL reaction tube (green)
Justification and evidence
The WHO Global TB Programme has initiated an update of the current guidelines and commissioned a systematic review on the use of Xpert MTB/RIF and Xpert Ultra for the diagnosis of TB in people with signs and symptoms of TB.
The population, intervention, comparator and outcome (PICO) questions were designed to form the basis for the evidence search, retrieval and analysis.

The systematic reviews were conducted to summarize the current literature on the diagnostic accuracy of Xpert MTB/RIF and Xpert Ultra for the diagnosis of TB and rifampicin resistance. This was done as part of the WHO process to develop updated guidelines for the use of molecular assays intended as initial tests for the diagnosis of pulmonary and extrapulmonary TB in adults and children. The data on children, where possible, were reported separately from adults.
The certainty of the evidence was assessed consistently through PICO questions, using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which produces an overall quality assessment (or certainty) of evidence and a framework for translating evidence into recommendations. The certainty of the evidence is rated as high, moderate, low or very low. These four categories “imply a gradient of confidence in the estimates” (10). In the GRADE approach, even if diagnostic accuracy studies are of observational design, they start as high-quality evidence.
At least two review authors independently completed the quality assessment of diagnostic accuracy studies (QUADAS)-2 assessments. Any disagreements were resolved through discussion or consultation with a third review author.
Finally, where applicable, meta-analyses were performed to estimate pooled sensitivity and specificity separately for Xpert MTB/RIF and Xpert Ultra, and separately for TB (either pulmonary or extrapulmonary) and rifampicin resistance.
Data synthesis was structured around the preset PICO questions list below. Details of studies included in the current analysis are given in Web Annex 1.1: Xpert MTB/RIF and Xpert Ultra. Summary of the results and details of the evidence quality assessment are available in Web Annex 2.1: Xpert MTB/RIF and Xpert Ultra.
PICO 1: Among adults with signs and symptoms of pulmonary TB, seeking care at health care facilities, should Xpert MTB/RIF / Xpert Ultra be used as an initial test for diagnosis of pulmonary TB and rifampicin resistance?
1.1 What is the impact of Xpert MTB/RIF on patient-important outcomes (cure, mortality, time to diagnosis and time to start treatment)?
The aim of the review was to assess the impact on patient-important outcomes of diagnostic strategies using Xpert MTB/RIF compared with strategies using smear microscopy. The following outcomes were considered: all-cause mortality, pretreatment loss to follow-up, cure, time to diagnosis and time to treatment initiation.
For the impact of Xpert MTB/RIF on patient-important outcomes for TB, seven studies were included (16 421 participants): two individually randomized trials (Mupfumi 2014; Theron 2014), four cluster randomized trials (Churchyard 2015; Cox 2014; Ngwira LG 2017; Durovni 2014), and one individual patient data (IPD) meta-analysis (Di Tanna 2019) (see Web Annex 1.1: Xpert MTB/RIF and Xpert Ultra for details of these and other studies). All studies were conducted in high TB burden and high TB/HIV burden countries. There were two trials in South Africa (Churchyard 2015; Cox 2014), one in Zimbabwe (Mupfumi 2014), one in Malawi (Ngwira LG 2017), one in Brazil (Durovni 2014) and two multicountry studies with sites in South Africa, United Republic of Tanzania, Zambia and Zimbabwe (Theron 2014, Di Tanna 2019). All studies were conducted in outpatient settings and enrolled participants aged 18 years or older.
Web Annex 4.1: Impact of diagnostic test Xpert MTB/RIF on patient-important outcomes for tuberculosis: a systematic review.
1.2 What is the diagnostic accuracy of Xpert MTB/RIF for pulmonary TB and rifampicin resistance, as compared with MRS?
The aim of the review was to assess the diagnostic accuracy of Xpert MTB/RIF for pulmonary TB and rifampicin resistance in adults. Randomized trials, cross-sectional studies and cohort studies were included, using respiratory specimens that evaluated Xpert MTB/RIF alone or together with Xpert Ultra against the reference standards of culture for TB detection and culture-based DST or MTBDRplus for rifampicin resistance. Only studies that enrolled adults (aged >15 years) were eligible. For the evaluation of TB detection, studies were included that evaluated the index tests in people with signs and symptoms of pulmonary TB, except for studies in PLHIV, where studies were eligible for inclusion irrespective of signs and symptoms of pulmonary TB (e.g. studies that performed TB screening in PLHIV as part of intensified case finding or before TB preventive therapy).
For detection of pulmonary TB, a total of 94 studies were identified. Of these, 85 studies (40 652 participants) evaluated Xpert MTB/RIF and nine studies (3881 participants) evaluated both Xpert Ultra and Xpert MTB/RIF. Of the 94 studies, 50 (53%) took place in high TB burden and 54 (57%) in high TB/HIV burden countries. Most studies had low risk of bias. Also, most studies had low concern about applicability because participants in these studies were evaluated in primary care facilities, local hospitals or both settings.
For detection of rifampicin resistance, 57 studies (8287 participants) evaluated Xpert MTB/RIF. Of the 57 studies, 27 took place in high MDR-TB burden countries. Most studies were judged as having low risk of bias.
Web Annex 4.2: Xpert MTB/RIF and Xpert Ultra for detecting active tuberculosis in adults with signs and symptoms of pulmonary TB: an updated systematic review.
1.3 What is the diagnostic accuracy of Xpert Ultra for pulmonary TB and rifampicin resistance, as compared with MRS?
For detection of pulmonary TB, a total of nine studies (3881 participants) evaluated both Xpert Ultra and Xpert MTB/RIF. For Xpert Ultra, a composite reference standard was also used that included clinical components as defined by the primary study authors. For detection of rifampicin resistance, eight studies (1039 participants) evaluated Xpert Ultra. The total number of Xpert Ultra studies includes one study that provided data for two cohorts; therefore, we classified these as two distinct studies, Mishra 2019a and Mishra 2019b. Most studies were judged as having high certainty of evidence.
Web Annex 4.2: Xpert MTB/RIF and Xpert Ultra for detecting active tuberculosis in adults with signs and symptoms of pulmonary TB: an updated systematic review.
PICO 2: Among children with signs and symptoms of pulmonary TB, seeking care at health care facilities, should Xpert MTB/RIF / Xpert Ultra be used as an initial test for diagnosis of pulmonary TB and rifampicin resistance?
2.1 What is the diagnostic accuracy of Xpert MTB/RIF for pulmonary TB and rifampicin resistance in children, as compared with MRS and CRS?
The initial search resulted in 835 individual records, with one additional reference identified through other sources, giving a total of 836 records, from which 707 were excluded. Initially, the remaining 129 articles were retrieved. After full-text review, 50 studies were included in the quantitative meta-analysis; of these, 40 (80%) took place in high TB burden countries and 10 in high TB/HIV burden countries. For pulmonary TB detection, 43 studies were included that evaluated the diagnostic accuracy of Xpert MTB/RIF in children, and three that evaluated both Xpert Ultra and Xpert MTB/RIF. Forty-two studies evaluated pulmonary TB using a reference standard of culture, and one study evaluated pulmonary TB using smear microscopy only.
In terms of methodological quality, in the patient selection domain, most studies (83%) evaluating pulmonary TB were judged to have low risk of bias. In the index test domain, all studies were judged to have low risk of bias. In the flow and timing domain, most studies (88%) were judged to have low risk of bias. In the reference standard domain, with respect to the MRS, 47% of studies were judged to have unclear risk of bias because only one culture was used to exclude TB. With respect to the composite reference standard, all studies were judged to have unclear risk of bias because of imperfect accuracy of the composite reference standard and differing definitions of this standard used by the primary study authors. Regarding applicability, in the patient selection domain, 50% of studies were judged as having high or unclear risk of bias, because participants were evaluated exclusively as inpatients at tertiary care centres, or the clinical setting was unclear. With respect to applicability of the index test, most studies (72%) were judged as having low concern owing to standardized application of the index tests. Eleven studies evaluating stool as a specimen for Xpert MTB/RIF or Xpert Ultra were judged to have unclear risk of bias because of the absence of a standardized protocol for stool preparation. Applicability of the reference standard was considered as a low concern for most studies (93%).
To generate evidence about the detection of rifampicin resistance, six studies were included. All of the six studies (223 participants) evaluated only Xpert MTB/RIF and were conducted in high TB burden countries and in high MDR-TB burden countries. Among the studies, 50% had a low risk of bias with respect to patient selection, while all studies had a low risk of bias with respect to the reference standard. Risk of bias was considered low for the reference standard if an automated process was used or it was clear that the reference standard results were interpreted without knowledge of the index tests. For all six studies, there were applicability concerns regarding patient selection because of enrolment exclusively from inpatient or tertiary centres.
For the meta-analysis, a total of 23 studies (6612 participants) evaluated sputum specimens; 14 studies (3468 participants) evaluated gastric specimens; four studies (1125 participants) evaluated nasopharyngeal specimens; and 11 studies (1592 participants) evaluated stool specimens – all of these studies evaluated Xpert MTB/RIF alone. Three studies (753 participants) evaluated both Xpert MTB/RIF and Xpert Ultra on frozen sputum specimens. One study (195 participants) evaluated both Xpert MTB/RIF and Xpert Ultra on nasopharyngeal specimens.
2.2 What is the diagnostic accuracy of Xpert Ultra for pulmonary TB and rifampicin resistance in children, as compared with MRS and CRS?
No studies evaluated Xpert Ultra alone. Three studies (753 participants) evaluated both Xpert MTB/RIF and Xpert Ultra on frozen sputum specimens. One study (195 participants) evaluated both Xpert MTB/RIF and Xpert Ultra on nasopharyngeal specimens.
Web Annex 4.4: Xpert MTB/RIF and Xpert Ultra for detecting active tuberculosis in children: an updated systematic review.
PICO 3: Among adults with signs and symptoms of extrapulmonary TB, seeking care at health care facilities, should Xpert MTB/RIF / Xpert Ultra be used as an initial test for diagnosis of extrapulmonary TB and rifampicin resistance?
3.1 What is the diagnostic accuracy of Xpert MTB/RIF for extrapulmonary TB and rifampicin resistance in adults, as compared with MRS and CRS?
There are difficulties in obtaining extrapulmonary specimens both from children and adults, and technical limitations of conventional bacteriological methods to aid diagnosis. Thus, various non-pulmonary specimens and composite reference standards are often used in evaluating the performance of new diagnostic technologies in extrapulmonary TB.
For detection of extrapulmonary TB, 65 studies were included. A total of 63 studies (13 144 participants) evaluated Xpert MTB/RIF, including five that evaluated both Xpert MTB/RIF and Xpert Ultra. The included studies evaluated Xpert MTB/RIF in cerebrospinal fluid (CSF) specimens comprising lymph node aspirate, lymph node biopsy, pleural fluid, urine, synovial fluid, peritoneal fluid, pericardial fluid and blood.
Of the total of 65 studies, 39 (60%) took place in high TB burden and 41 (63%) in high TB/HIV burden countries. Risk of bias was judged to be low in the domains of patient selection, index test, and flow and timing; and high or unclear in the reference standard domain because many studies decontaminated sterile specimens before culture inoculation. Regarding applicability, in the patient selection domain, high or unclear concern was expressed for most studies because either the participants were evaluated exclusively as inpatients at tertiary care centres, or the clinical settings were unclear.
Annex 4.3: Xpert MTB/RIF and Xpert Ultra for detecting active tuberculosis in adults with signs and symptoms of extrapulmonary TB: an updated systematic review.
3.2 What is the diagnostic accuracy of Xpert Ultra for extrapulmonary TB and rifampicin resistance in adults, as compared with MRS?
Six studies (507 participants) evaluated Xpert Ultra for the detection of extrapulmonary TB. The included studies evaluated the test in CSF specimens comprising lymph node biopsy, pleural fluid, urine and synovial fluid. Serious concerns were expressed regarding the indirectness of the evidence; these concerns related to applicability (i.e. evidence was generated in tertiary referral medical centres), and imprecision of the evidence, related mostly to low numbers of participants included in studies. Certainty of evidence was generally judged as being between low and very low.
Web Annex 4.3: Xpert MTB/RIF and Xpert Ultra for detecting active tuberculosis in adults with signs and symptoms of extrapulmonary TB: an updated systematic review.
PICO 4: Among children with signs and symptoms of extrapulmonary TB and rifampicin resistance, seeking care at health care facilities, should Xpert MTB/RIF / Xpert Ultra be used as an initial test for diagnosis of extrapulmonary TB and rifampicin resistance?
4.1 What is the diagnostic accuracy of Xpert MTB/RIF for extrapulmonary TB and rifampicin resistance in children, as compared with MRS and CRS?
4.2 What is the diagnostic accuracy of Xpert Ultra for extrapulmonary TB and rifampicin resistance in children, as compared with MRS and CRS?
To evaluate detection of extrapulmonary TB, studies that evaluated the diagnostic accuracy of Xpert MTB/RIF in children with signs or symptoms of lymph node TB or TB meningitis were included.
For diagnosis of lymph node TB, six studies (210 participants) evaluated Xpert MTB/RIF against an MRS of smear or culture on lymph node specimens. Two studies (105 participants) evaluated Xpert MTB/RIF against a composite reference standard for lymph node TB. For TB meningitis, six studies (241 participants) evaluated Xpert MTB/RIF against culture on CSF. In addition, two studies (155 participants) assessed Xpert MTB/RIF against a composite reference standard that included a clinical diagnosis of TB meningitis. The certainty of evidence was judged to be very low for sensitivity, and low for specificity of detection of both TB meningitis and lymph node TB.
No studies evaluating the accuracy of Xpert Ultra for detecting lymph node TB or TB meningitis were identified.
Web Annex 4.4: Xpert MTB/RIF and Xpert Ultra for detecting active tuberculosis in children: an updated systematic review.
PICO 5: Among people with signs and symptoms of pulmonary TB, seeking care at health care facilities, do repeated Xpert (Ultra) tests on subsequent samples as an initial test for diagnosis of pulmonary TB and rifampicin resistance increase sensitivity/ specificity compared with a single initial test?
5.1 Xpert Ultra repeated test for the diagnosis of pulmonary TB in adults with signs and symptoms of pulmonary TB who have an initial Xpert Ultra trace result, as compared with MRS?
For adults, with initial Xpert Ultra trace results, three studies were identified: Mishra 2019a (4 participants), Piersimoni 2019 (4 participants), and Dorman 2018 (42 participants) (see Web Annex 1.1: Xpert MTB/RIF and Xpert Ultra for details of included studies). Piersimoni 2019 retested the same initial sample, whereas Dorman 2018 retested a separately collected sputum sample. Mishra 2019a retested only those participants with discrepant results (i.e. Ultra trace positive/culture negative), and retested new specimens obtained a median of 444 days (range 245–526 days) after initial testing. Owing to limited data, a meta-analysis was not performed. The evidence was downgraded one level for inconsistency and two levels for imprecision. Serious concerns were expressed for inconsistency, and very serious concerns for imprecision. Certainty of evidence was judged to be very low for both sensitivity and specificity.
Web Annex 4.2: Xpert MTB/RIF and Xpert Ultra for detecting active tuberculosis in adults with signs and symptoms of pulmonary TB: an updated systematic review.
5.2 More than one Xpert MTB/RIF versus one Xpert MTB/RIF to diagnose pulmonary TB in children with signs and symptoms of pulmonary TB, as compared with MRS?
For children, five studies (2119 participants) were included that have evaluated the diagnostic accuracy of multiple Xpert MTB/RIF tests compared with a single test. Serious concerns were expressed for indirectness, because patients were enrolled from inpatient tertiary care settings, which could lead to the enrolment of children with more advanced disease. Also, serious concerns were expressed for imprecision, related to the low number of children with pulmonary TB contributing to this analysis for the observed sensitivity. Overall, the certainty of evidence was judged to be very low for sensitivity and moderate for specificity.
Web Annex 4.4: Xpert MTB/RIF and Xpert Ultra for detecting active tuberculosis in children: an updated systematic review.
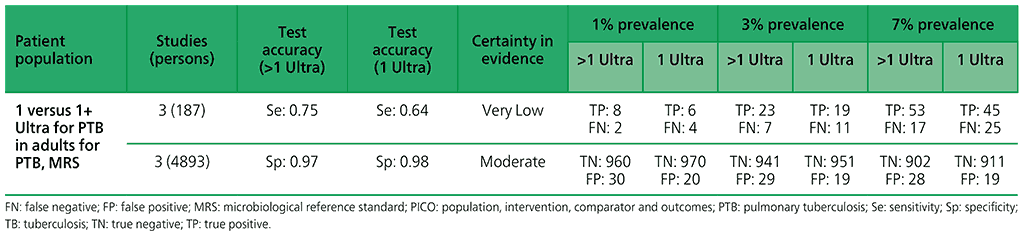
5.3 More than one Xpert Ultra versus one Xpert Ultra to diagnose pulmonary TB in children with signs and symptoms of pulmonary TB, as compared with MRS?
For children, one study (163 participants) was included that evaluated the diagnostic accuracy of multiple Xpert Ultra tests in sputum compared with a single test. The certainty of evidence was judged to be very low for sensitivity and low for specificity owing to serious concerns for indirectness and imprecision. In addition, one study (130 participants) was included that evaluated the diagnostic accuracy of multiple Xpert Ultra tests in nasopharyngeal aspirates compared with a single test. Overall, the certainty of evidence was judged to be very low both for sensitivity and specificity, owing to very serious concerns for indirectness and imprecision.
Web Annex 4.4: Xpert MTB/RIF and Xpert Ultra for detecting active tuberculosis in children: an updated systematic review.
PICO 6: Among adults either with signs and symptoms of TB or chest radiograph with lung abnormalities suggestive of pulmonary TB or both, should Xpert MTB/RIF or Xpert Ultra alone be used to define a case of active TB disease (10)?
The aim of the review was to assess the diagnostic accuracy of Xpert MTB/RIF and Xpert Ultra for pulmonary TB in adults (aged ≥15 years) among the general population. Data from four nationally representative and two subnational prevalence surveys for active TB disease, cross-sectional in design, were included. These surveys used sputum samples that evaluated Xpert MTB/RIF or Xpert Ultra against the reference standard of culture for TB. For the evaluation of TB detection, the surveys evaluated the index tests in adults (aged ≥15 years) with chest X-ray abnormalities or symptoms suggestive of pulmonary TB (or both). For detection of pulmonary TB, a total of six surveys were identified.
6.1 Xpert MTB/RIF to diagnose pulmonary TB in adults in the general population with signs and symptoms of pulmonary TB or chest radiograph with lung abnormalities or both, as compared with MRS?
The analysis reported on the results of four surveys, including 49 556 participants. Assessment of the quality of the evidence revealed serious deficiencies in the evidence quality.
Indirectness: the populations in these prevalence surveys differed from the general population with respect to prior testing (e.g. symptom screen was limited to cough for 14 days or more) and the availability of results of both symptom screen and chest radiography in most participants included in the studies. The evidence was downgraded one level for indirectness.
Inconsistency: the sensitivity estimate for Bangladesh was 84%, which was higher than the sensitivity estimates for the other three countries (range, 68–69%). Lower HIV prevalence in Bangladesh could only partly explain the inconsistency. The evidence was downgraded one level for inconsistency. Overall, the certainty of evidence was judged to be low for sensitivity and moderate for specificity.
6.2 Xpert Ultra to diagnose pulmonary TB in adults in the general population with signs and symptoms of pulmonary TB or chest radiograph with lung abnormalities or both, as compared with MRS.
The analysis reported on the results of four surveys, including 11 488 participants. The included countries were Myanmar, South Africa (TREATS project) and Zambia (TREATS project). The average prevalence of TB in these countries was 2.8% (range 1.6–6.7%).
Indirectness: the populations in these prevalence surveys differed from the general population with respect to prior testing (e.g. symptom screen was limited to cough for 14 days or more) and the availability of results of both symptom screen and chest radiography in most participants included in the studies. The evidence was downgraded one level for indirectness
Imprecision: there were relatively few participants contributing to this analysis, and a wide 95% confidence interval (CI). The 95% CI around true positives and false negatives may lead to different decisions, depending on which limits are assumed. The evidence was downgraded one level for imprecision. Overall, the certainty of evidence was judged to be low for sensitivity and moderate for specificity.
6.3 Two Xpert Ultra versus one Xpert Ultra to diagnose pulmonary TB in adults in the general population with signs and symptoms of TB or chest radiograph with lung abnormalities or both, as compared with MRS.
The analysis reported on the results of three surveys, including 5080 participants. Serious concerns were expressed about the indirectness of the available evidence. This was because most of the data were from Myanmar, and the results may not be applicable to other settings. In addition, very serious concerns were expressed about imprecision because the analysis was based on data for only a small number of individuals. The 95% CIs for two Xpert Ultra assays and one Xpert Ultra assay were wide. Overall, the certainty of evidence was judged to be very low for sensitivity and moderate for specificity.
Performance of the molecular assays
Table 2.1.1. PICO 1.1: What is the impact of Xpert MTB/RIF on patient-important outcomes (e.g. cure, mortality, time to diagnosis and time to start treatment)?
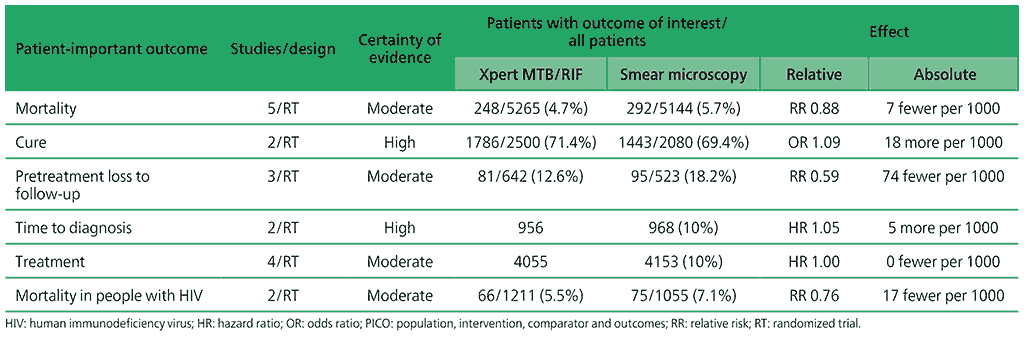
Table 2.1.2. PICO 1.2: What is the diagnostic accuracy of Xpert MTB/RIF for pulmonary TB in adults, as compared with MRS?
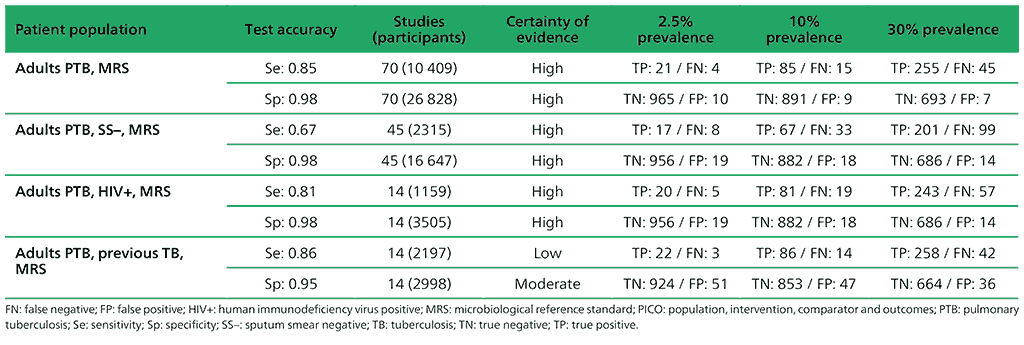
Table 2.1.3. PICO 1.2: What is the diagnostic accuracy of Xpert MTB/RIF for rifampicin resistance in adults with pulmonary TB, as compared with MRS?
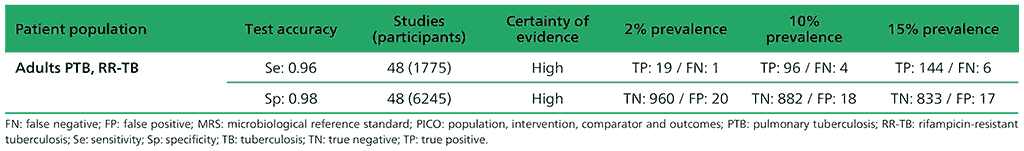
Table 2.1.4. PICO 1.3: What is the diagnostic accuracy of Xpert Ultra for pulmonary TB, as compared with MRS?
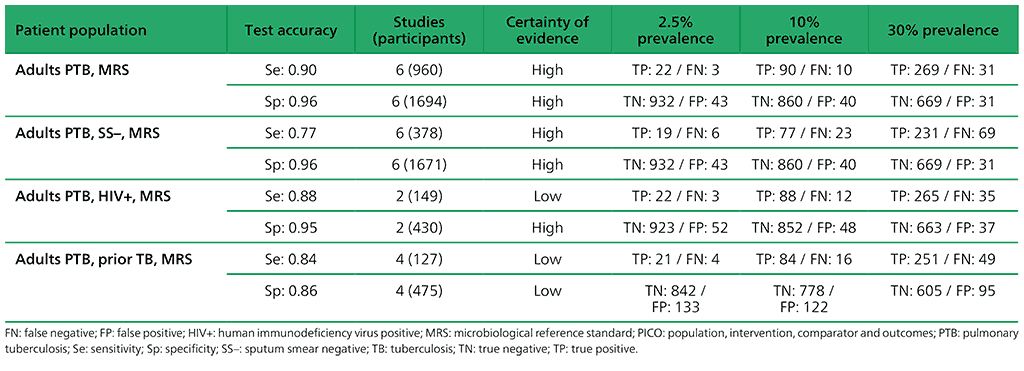
Table 2.1.5. PICO 1.3: What is the diagnostic accuracy of Xpert Ultra for rifampicin resistance in adults with pulmonary TB, as compared with MRS?
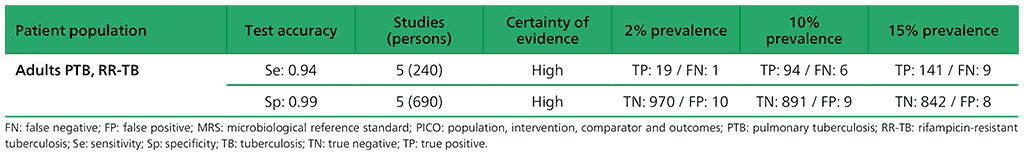
Table 2.1.6. PICO 2.1: What is the diagnostic accuracy of Xpert MTB/RIF for pulmonary TB in children, as compared with MRS and CRS?
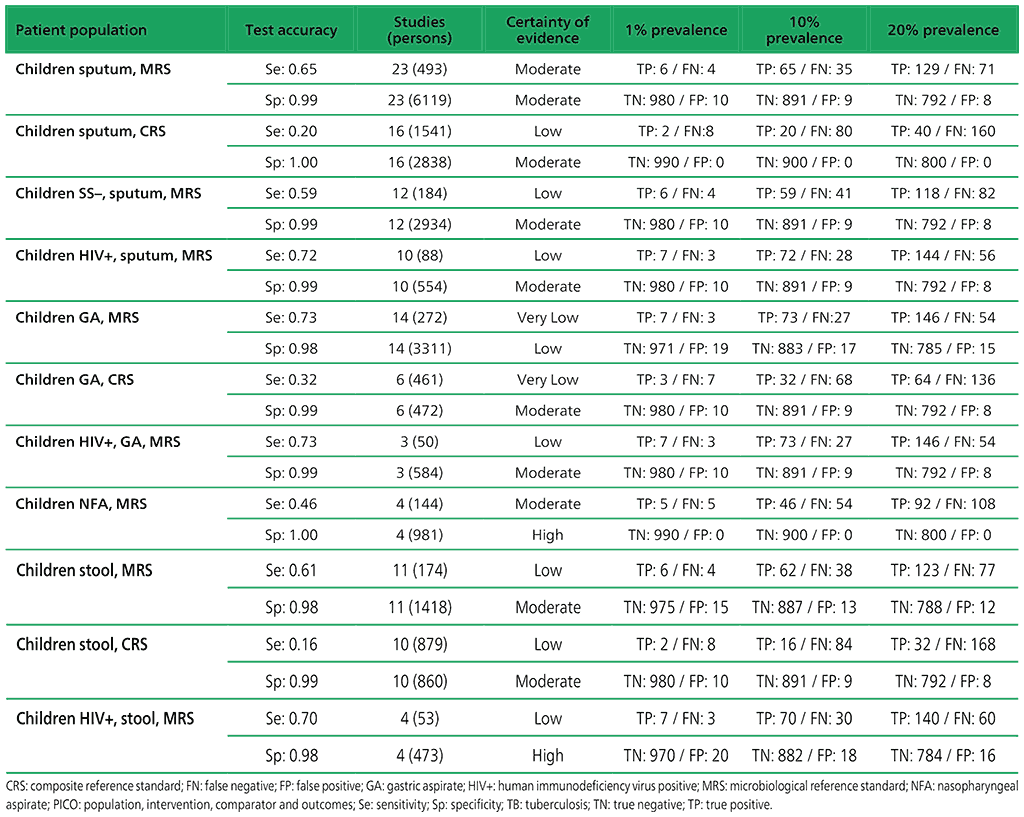
Table 2.1.7. PICO 2.1: What is the diagnostic accuracy of Xpert MTB/RIF for rifampicin resistance in children, as compared with MRS?
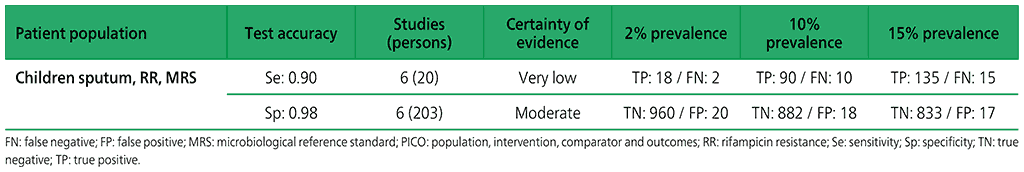
Table 2.1.8. PICO 2.2: What is the diagnostic accuracy of Xpert Ultra for pulmonary TB in children, as compared with MRS and CRS?
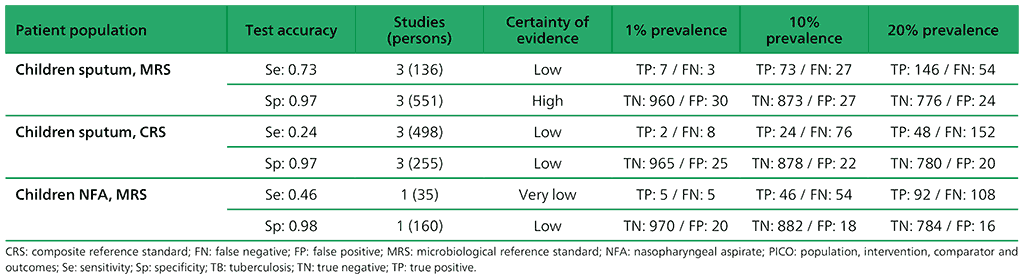
Table 2.1.9. PICO 3.1: What is the diagnostic accuracy of Xpert MTB/RIF for extrapulmonary TB in adults, as compared with MRS and CRS?
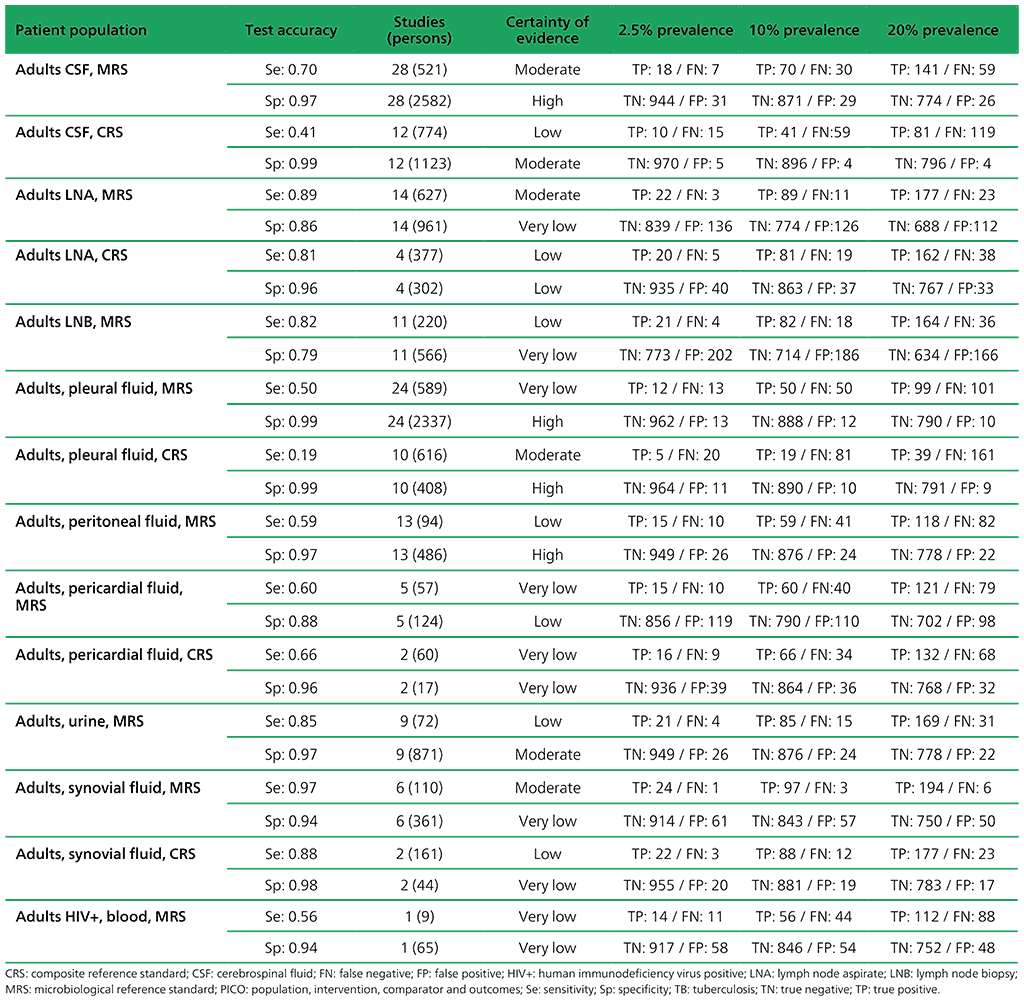
Table 2.1.10. PICO 3.1: What is the diagnostic accuracy of Xpert MTB/RIF for rifampicin resistance in adults with extrapulmonary TB, as compared with MRS?
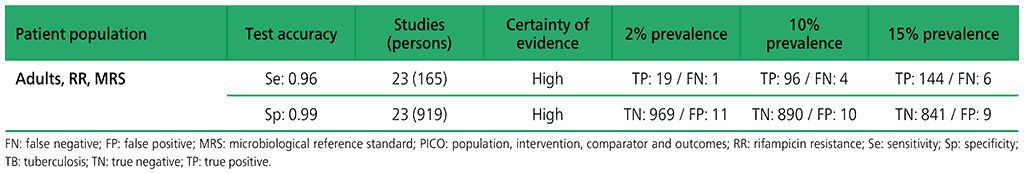
Table 2.1.11. PICO 3.2: What is the diagnostic accuracy of Xpert Ultra for extrapulmonary TB in adults, as compared with MRS and CRS?
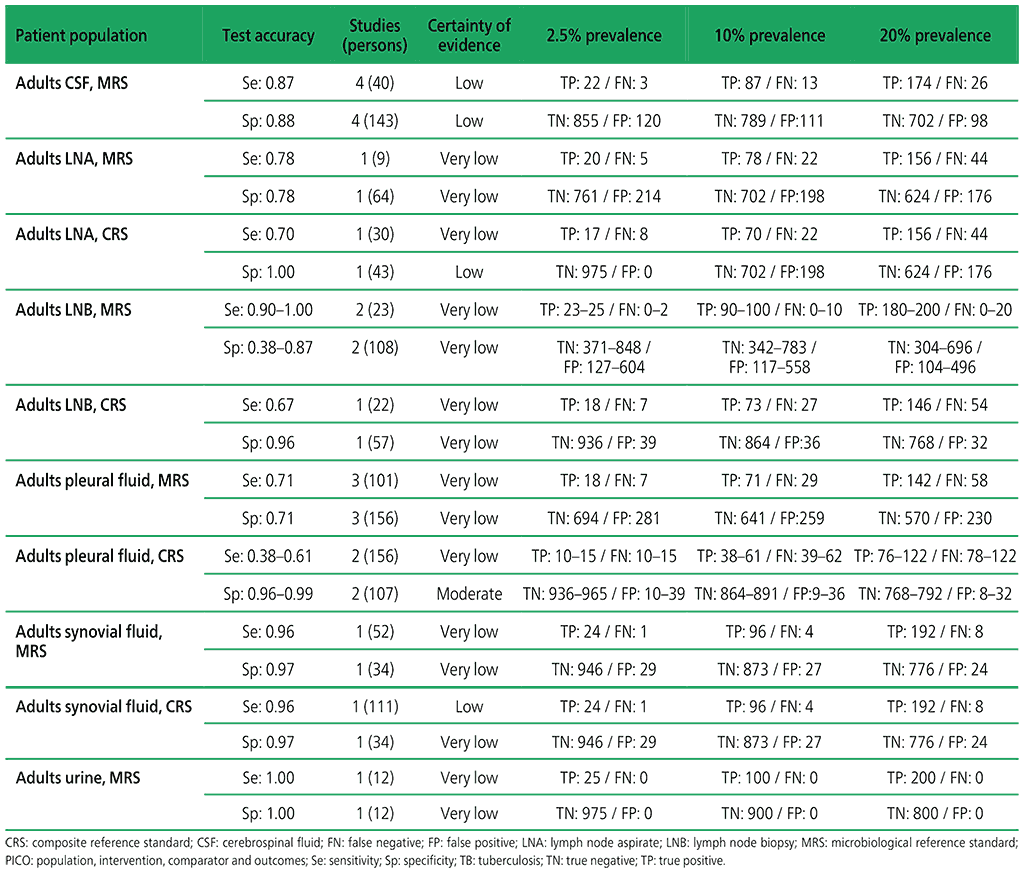
Table 2.1.12. PICO 3.2: What is the diagnostic accuracy of Xpert Ultra for rifampicin resistance in adults with extrapulmonary TB, as compared with MRS and CRS?
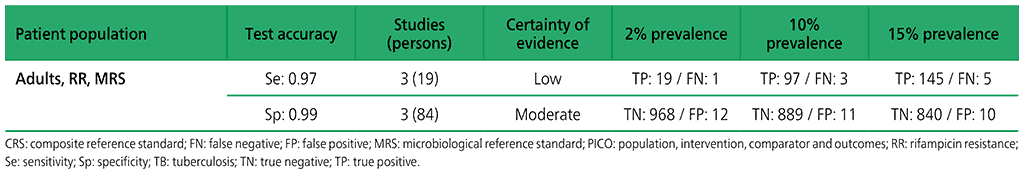
Table 2.1.13. PICO 4.1: What is the diagnostic accuracy of Xpert MTB/RIF for extrapulmonary TB in children, as compared with MRS?
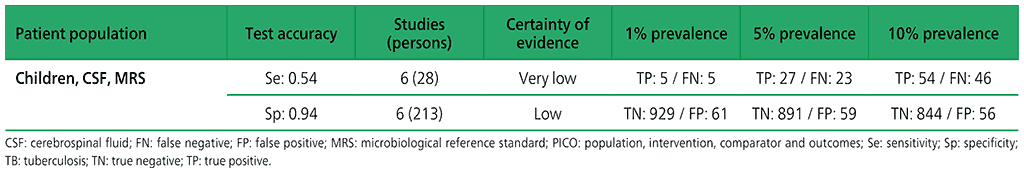
Table 2.1.14. PICO 5.1: Xpert Ultra repeated test for the diagnosis of pulmonary TB in adults with signs and symptoms of pulmonary TB who have an initial Ultra trace result, as compared with MRS?
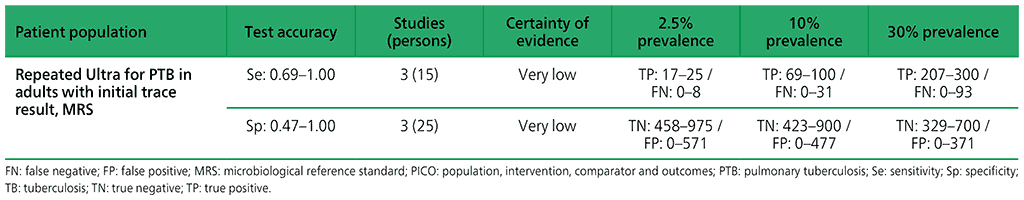
Table 2.1.15. PICO 5.2: More than one Xpert MTB/RIF versus one Xpert MTB/RIF to diagnose pulmonary TB in children with signs and symptoms of pulmonary TB, as compared with MRS?
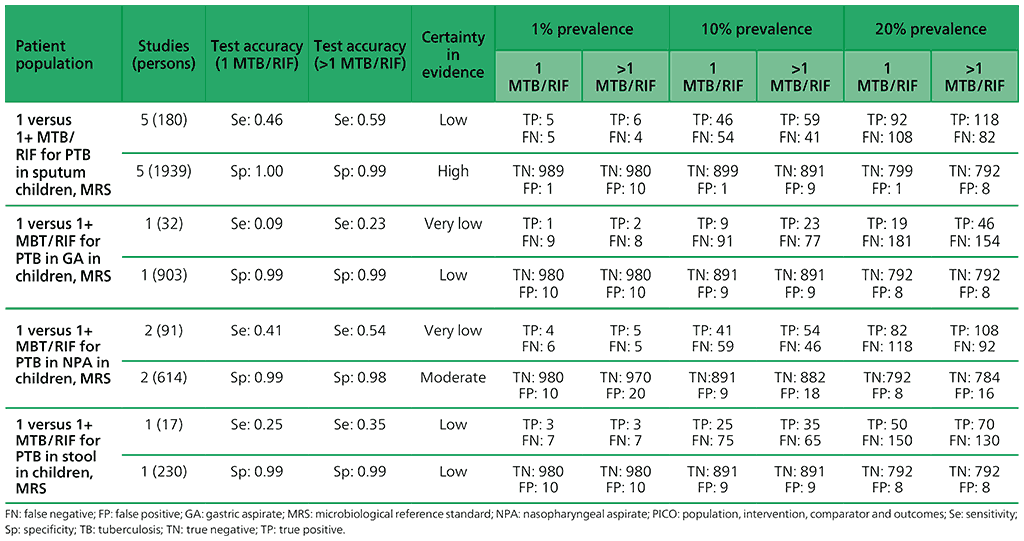
Table 2.1.16. PICO 5.3: More than one Xpert Ultra versus one Xpert Ultra to diagnose pulmonary TB in children with signs and symptoms of pulmonary TB, as compared with MRS?
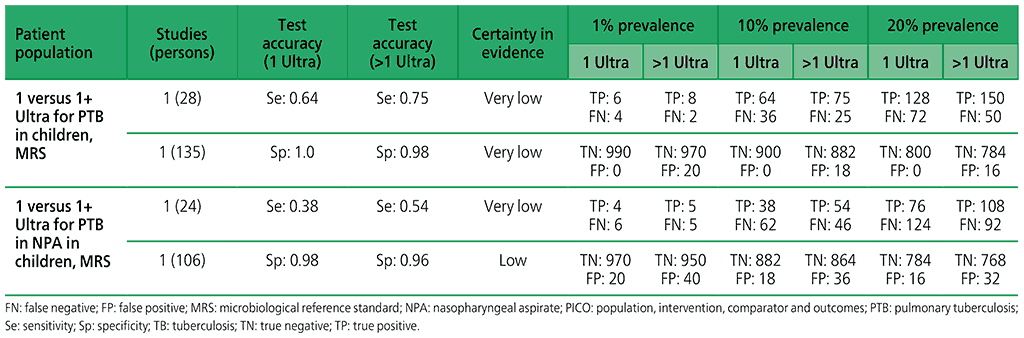
Table 2.1.17. PICO 6.1–6.2: Among adults in the general population with signs and symptoms of pulmonary TB or chest radiograph with lung abnormalities or both, should Xpert MTB/RIF or Xpert Ultra alone be used to define a case of active TB disease, as compared with MRS?
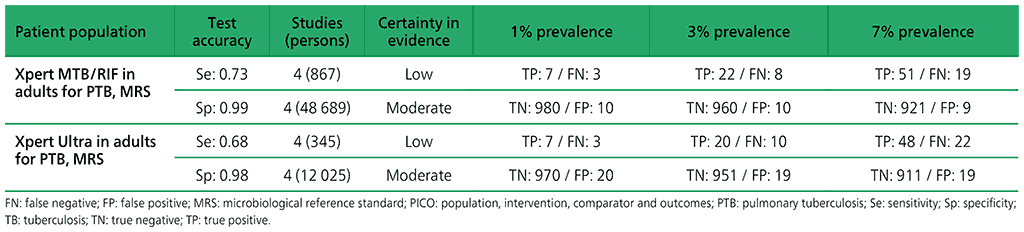
Table 2.1.18. PICO 6.3: Two Xpert Ultra versus one Xpert Ultra to diagnose pulmonary TB in adults in the general population with signs and symptoms of TB or chest radiograph with lung abnormalities or both, as compared with MRS.
Cost–effectiveness analysis
This section deals with the following additional question:
What are the comparative cost, affordability and cost–effectiveness of implementation of Xpert MTB/RIF, Xpert Ultra?
A systematic review was carried out, focusing on economic evaluations of molecular-based tests for the diagnosis of active TB. The tests included GeneXpert MTB/RIF (referred to as Xpert MTB/RIF) and the novel Xpert Ultra. The objective of the review was to summarize current economic evidence and further understand the costs, cost–effectiveness and affordability of these molecular tests for TB diagnosis. Twenty-eight studies were identified that met the inclusion criteria and addressed one of the PICO questions of interest. No studies assessing the cost–effectiveness of Xpert Ultra. Most of the studies assessed Xpert MTB/RIF in outpatient settings in countries in Africa; however, also included were studies among outpatients and hospitalized patients in other countries, such as Brazil, China, Germany, Hong Kong Special Administrative Region (SAR), India, South Africa and the USA.
Studies employed a variety of different modelling approaches, populations and settings. The included studies varied in their costing, effectiveness and epidemiological parameters, making direct comparisons across studies challenging. Furthermore, variations in what costing elements, implementation costs and downstream costs were included in the different studies.
Although many studies demonstrated that Xpert MTB/RIF may be cost effective in diagnosing pulmonary TB, key implementation conditions and settings had a strong effect on cost– effectiveness and must be considered when implementing this test. The cost–effectiveness of Xpert MTB/RIF was shown to be improved among certain populations: those with higher TB prevalence, in PLHIV and those where rates of empirical treatment were low. Cost–effectiveness of Xpert MTB/RIF is strongly affected by factors such as the location of GeneXpert machines (i.e. centralized versus decentralized facilities), test volume, underlying TB prevalence, level of empirical treatment and pretreatment loss to follow-up.
Caution should be used when generalizing cost–effectiveness and economic evaluations across settings. Local implementation conditions and settings should be taken into account, and local implementation studies may be helpful to assess the likely impact on case finding, long-term outcomes and cost–effectiveness.
There is substantial economic evidence around the implementation and scale-up of Xpert MTB/RIF in different settings, most notably among outpatients presenting with signs and symptoms of TB. Most of these studies found that Xpert MTB/RIF would probably be cost effective. Still, there were some exceptions, and it was clear that differences in implementation approaches and settings could have an important impact on cost–effectiveness. Studies employed a wide variety of modelling and analysis approaches, assumptions, diagnostic algorithms, and comparators. They also assessed different study settings, making comparisons across studies and generalizations to other challenging settings.
Studies highlighted that implementation factors and settings need to be considered when generalizing cost–effectiveness results to different settings. Important factors in determining whether Xpert MTB/RIF may be cost effective in any given setting include current standard of care, level of empirical treatment, existing testing facilities, location of Xpert MTB/RIF (centralized or decentralized facilities), TB prevalence, patient volume, pretreatment loss to follow-up and existing linkage to care. Other important cost components include whether implementation costs associated with Xpert MTB/RIF scale-up are considered and whether downstream costs (e.g. for TB and MDR-TB treatment, and antiretroviral therapy and HIV care) were included.
Web Annex 4.5: Systematic literature review of economic evidence for molecular assays intended as initial tests for the diagnosis of pulmonary and extrapulmonary TB in adults and children.
User perspective
This section deals with the following question:
Are there implications for feasibility, accessibility, patient equity and human rights from the implementation of Xpert MTB/RIF, Xpert Ultra?
The results of the qualitative research show that participants place great value on the ability of Xpert¹² to improve the diagnosis of DR-TB; they also show the impact on patients if they cannot access testing for drug resistance through this technology. The impact on case notification and the value of Xpert for finding more TB cases was less clear, owing to widespread clinical treatment, the prolonged turnaround time for results, and the challenges with feasibility and use of Xpert.
Although access has improved, not everybody who needs it can access Xpert testing. Simple laboratory procedures do not automatically translate into feasibility to implement. Instead, the feasibility of Xpert testing depends on government commitment to ensure functioning infrastructure and stable power, supply of cartridges and functioning laboratory services, investment in expertise for handling (discordant) results, effective repair services, staff with monitoring capacities, functioning sample transport, sustainable funding models and transparent donor agreements, and simple diagnostic algorithms.
Concerning acceptability, although Xpert has eased laboratory work through convenience and automation, the preference for Xpert in the laboratory can have undesired consequences for treatment monitoring with microscopy, and for reverting to microscopy if GeneXpert instruments become non-functional. Clinicians’ confidence in Xpert results is relatively high, but the challenges with feasibility and use mean that clinicians are at times deterred from ordering Xpert tests.
Summary of the results
- Xpert is unable to bridge disconnects or lack of capacity in general laboratory services. Participants valued the option to use a specimen other than sputum, but having GeneXpert machines available in the public sector does not necessarily mean that facilities and capacities are available to extract and make use of those specimens. For example, services for histopathology and bacteriology in one country may be disconnected, and sending a specimen to histopathology in the private sector, for instance, may mean that the sample will not return to a public sector GeneXpert machine.
- Xpert Ultra trace results complicate decision-making. Laboratory and clinical management of trace results was rarely straightforward. Study participants reported challenges with obtaining a second fresh sample when patients had left the facility or had been put on treatment and could not easily produce sputum. If repeat tests are conducted after trace, they cause confusion if the second test has a different result (e.g. is negative). Some laboratory managers are unsure which result to report, and clinicians need expertise and experience to conduct more extensive evaluation for trace patients. This presents challenges in peripheral settings and where turnaround times of confirmatory tests (e.g. phenotypic DST and LPA) slow down clinical decision-making.
- Discordant results of repeat tests and confirmatory tests can cause confusion around what should be considered the gold standard. This is particularly the case when specimen quality might be poor. Understanding and contextualizing discordant results requires continuous training, experience and expertise.
- Establishing a thorough TB history of patients is uncommon, and “previously treated” is defined differently. This has implications for potential false positive results through Xpert testing. Clear guidance is needed on how to define previously treated patients, how to handle their Xpert results, and how to capture outcomes in national databases accurately.
- The lack of trained counsellors and of information provided to patients on diagnostics have negative implications. Patients may be unwilling to accept a diagnosis and invest time and money in clinic visits, follow-up tests and treatment. Patients need better quality counselling by health workers to continue with diagnostic journeys and treatment; such counselling should include information about diagnostic technology and considerations for follow-up testing.
- Persistent underuse of GeneXpert machines is compounded by the challenges of delays due to sample transport, module breakdown, stock-out of cartridges or complicated diagnostic algorithms. The presence of local Cepheid agents is key for repair. However, high workload and staff turnover, combined with infrastructure and environmental conditions, still cause frequent module breakdown, and repair work can be slow or services deemed insufficient. The challenges of cartridge stock-out lead to important delays and disruption of workflows, leading to underuse.
- Diagnostic algorithms that are simple to follow in a specific facility (e.g. test all those with presumptive TB) are more feasible and enhance use, but this simplicity depends on cost and supplies. Cartridge stock-outs or prohibitive costs can complicate diagnostic algorithms, making them less feasible to follow and further compounding underuse. In Uganda, Xpert testing eligibility criteria had to be temporarily restricted to particular patient groups because of cartridge shortages that complicated the algorithm.
- Current donor agreements with governments regarding introduction of new diagnostic technologies are not transparent enough for civil society to be able to hold accountable and follow-up. Involving civil society in negotiating agreements and social contracts at the national level and local facility levels can enhance accountability and the responsiveness of governments, leading to improved implementation processes and access to diagnostics.
Web Annex 4.6: Report on user perspectives on Xpert testing: results from qualitative research.
Research priorities
Evaluation of the impact of Xpert Ultra testing on patient-important outcomes (cure, mortality, time to diagnosis and time to start treatment).
Evaluation of the diagnostic accuracy of Xpert Ultra in gastric or stool specimens for pulmonary TB and extrapulmonary TB in children.
Evaluation of the combinatorial benefit of multiple specimen types. Limited data were suggesting that the combination of non-invasive specimens performs comparably with traditional gastric specimens or induced sputum specimens.
Additional operational and qualitative research to determine the best approach to lessinvasive specimen collection.
Implementation studies on a method of suction for nasopharyngeal aspiration that is appropriate for low-skill or low-resource environments.
Extensive operational research into the use of stool as a diagnostic specimen in terms of integration into normal diagnostic clinical pathways, definition of laboratory protocols that successfully balance ease of implementation and diagnostic performance, and the impact of stool testing on patient-important outcomes. A dearth of qualitative research identifies child and family preferences for and acceptability of comparative diagnostic approaches.
Identification of an improved reference standard that accurately defines TB disease in children and paucibacillary specimens because the sensitivity of all available diagnostics is suboptimal.
Development of new tools that correctly diagnose a higher proportion of child TB cases. Ideally, the new tools will be rapid, affordable, feasible, and acceptable to children and their parents.
Comparison of different tests, including Xpert MTB/RIF and Xpert Ultra, to determine which tests (or strategies) yield superior diagnostic accuracy. The preferred study design is when all participants receive all available diagnostic tests or are randomly assigned to receive a particular test. Studies should include children and HIV-positive people. Future research should acknowledge the concern associated with culture as a reference standard and consider ways to address this limitation.
Development of rapid point-of-care diagnostic tests for extrapulmonary TB. Research groups should focus on developing diagnostic tests and strategies that use readily available clinical specimens such as urine rather than specimens that require invasive procedures for collection.
Operational research to ensure that tests are used optimally in settings of intended use.
Summary of changes between the 2013 guidance and the 2020 update

6 Mortality, cure, pretreatment loss to follow-up, time to diagnosis, treatment and mortality in PLHIV.
7 Based on PICO questions 3 and 4.
8 In low prevalence settings the effect of the second test was less pronounced.
9 Based on PICO question 5.
10 Culture.
11 Positive culture or a clinical decision to initiate treatment for TB.
12 When not specified, this term applies to both Xpert MTB/RIF and Xpert Ultra.
 تعليق
تعليق