Book traversal links for 4. Testing for TB infection
The third step in the cascade of care for PMTPT is testing for TB infection. Confirmation of TB infection increases the probability that individuals targeted for TPT will benefit from it. There is, however, no gold standard test for diagnosing TB infection. The tests currently available are indirect and require the person to mount an immune response. A positive test result is not by itself a reliable indicator that the infection will progress to TB disease, while a negative test result does not rule out TB infection, given the possibility of false-negative test results among at-risk groups such as young children and people who were recently infected. National health authorities should decide how to use tests for TB infection within PMTPT in view of the uncertainty of test results, the balance of benefit to harm of testing before starting TPT and the logistics of procurement and testing in the context of the programme.
4.1 Tests for TB infection
The currently recommended tests for TB infection are TST, TBSTs and IGRA. These tests detect immune sensitization (type IV or delayed-type hypersensitivity) to mycobacterial protein antigens that occur after infection by M. tuberculosis. A test for TB infection cannot on its own differentiate between TB infection and TB disease. A diagnosis of TB infection must be complemented by a negative test for TB disease, through clinical evaluation, CXR and examination of sputum or another suitable specimen if the person is symptomatic, as per national policy.
A brief description is given below of the different tests for TB infection. Details of WHO-recommended assays are available in the WHO operational handbook on tuberculosis. Module 3: diagnosis; tests for tuberculosis infection (53).
4.1.1 Skin tests
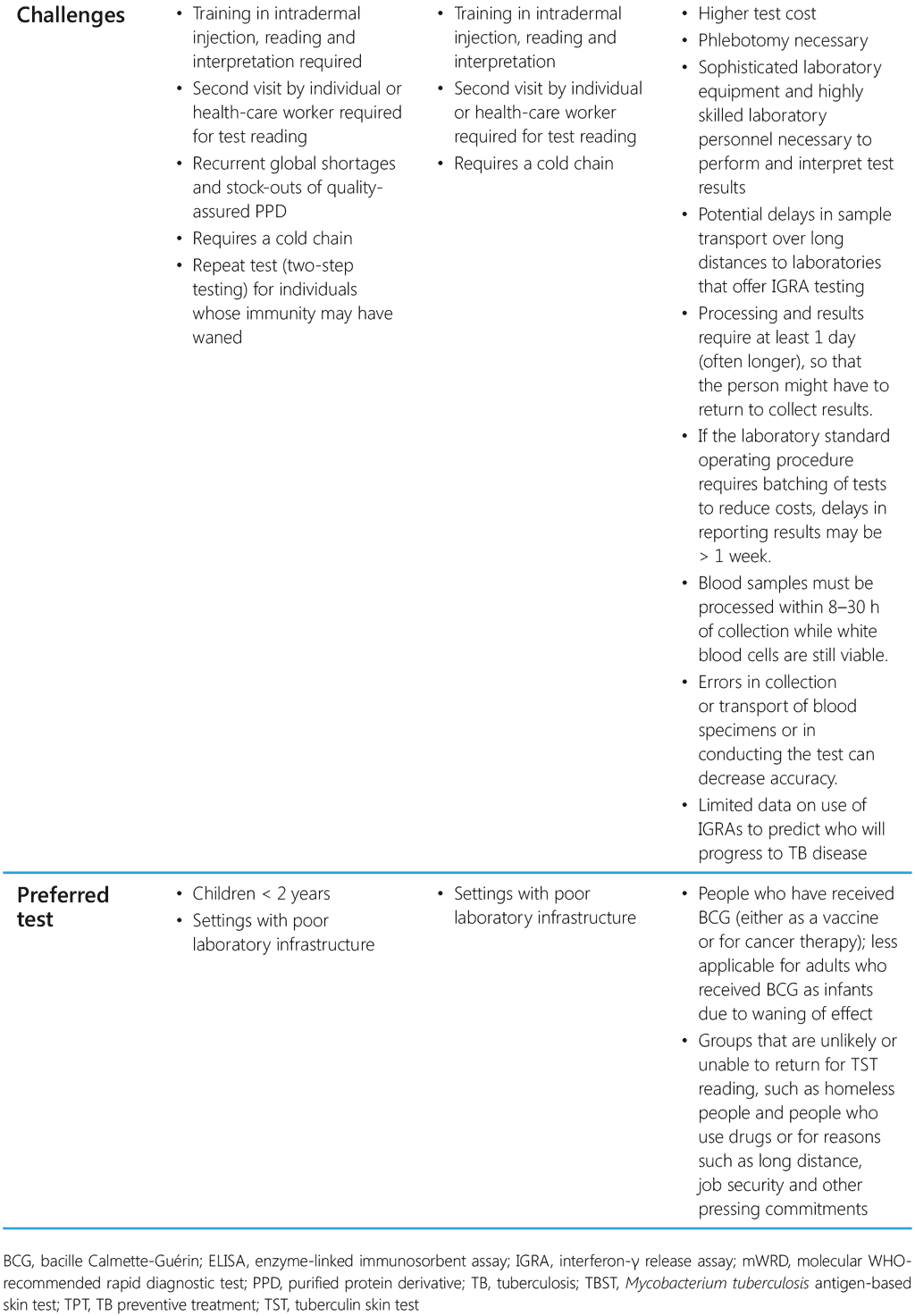
The TST measures delayed hypersensitivity reaction to exposure to purified protein derivative (PPD) of the mycobacterium. TBST is an in-vivo skin test for detection of TB infection with M. tuberculosis specific antigens, early secretory antigenic target 6 kDa protein and culture filtrate protein 10. Intradermal injection of PPD causes a delayed hypersensitivity reaction if a person has TB infection. The T cells in such individuals are sensitized by prior M. tuberculosis infection, and the injected agent recruits these immune cells to the skin test site, causing a local inflammatory reaction. Nurses and other health-care workers with the right training and skills can administer and read a TST or TBST. They should also be able to explain to the people tested what the result of the test implies in their case. There is no need for complex laboratory equipment or procedures or trained technicians to implement TST/TBST.
4.1.2 IGRAs
IGRAs are in-vitro blood tests for measuring interferon-γ released by circulating lymphocytes when mixed with M. tuberculosis antigens in an enzyme-linked immunosorbent assay (ELISA) or from the number of T-lymphocytes that produce interferon-γ (ELISPOT). IGRAs detect sensitization to M. tuberculosis by measuring interferon-γ release in response to antigens representing M. tuberculosis. IGRAs assess responses to synthetic overlapping peptides that represent specific M. tuberculosis proteins, such as early secretory antigenic target-6 and culture filtrate protein 10. These proteins are present in all M. tuberculosis complex species and stimulate measurable release of interferon-γ in most infected people but are absent from BCG vaccine strains and from most nontuberculous mycobacteria. For accurate measurement of an interferon-γ response, a fresh blood specimen that contains viable white blood cells is required.
4.1.3 Selecting a test for TB infection for programme use
TST, TBST or IGRA can be used to test for TB infection. There is no strong evidence that one test should be preferred over the others in terms of predicting progression from TB infection to TB disease. None of the tests should be used in people with low risks of TB infection and disease.
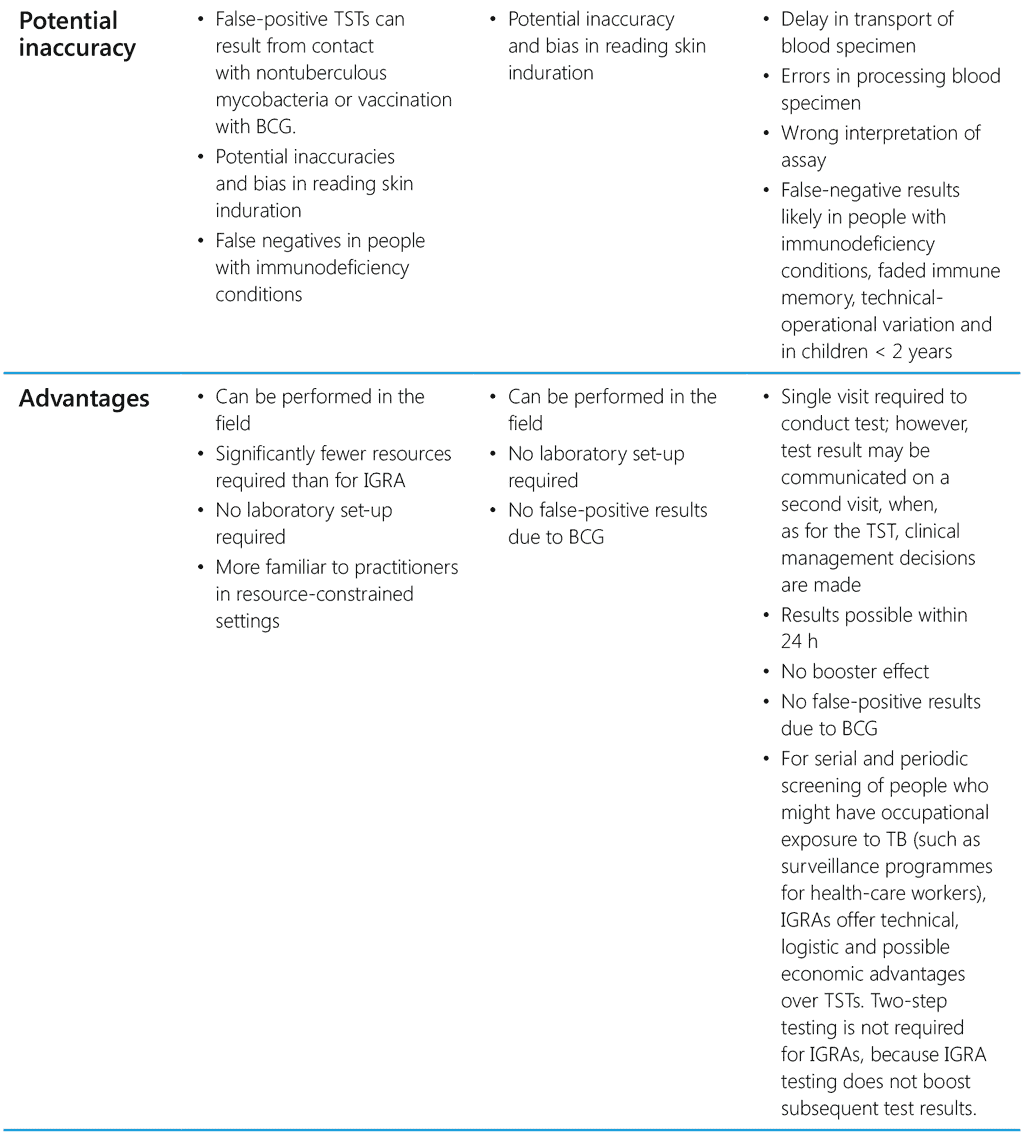
The choice of test for programmatic use depends on cost, availability, human resources and infrastructure to provide testing services. Table 2 summarizes the characteristic features and the advantages and disadvantages of currently available tests for TB infection.
Table 2. Characteristic features of TST, TBST and IGRA
4.2 Role of testing for TB infection
A decision on whether to test for TB infection before TPT is based on the expected prevalence of TB infection in the at-risk population, the risk of progression to TB disease and the risk of harm due to unnecessary TPT (Fig. 6). For individuals or populations at higher risk of harm due to TPT or a (relatively) lower risk of progression to TB disease, confirmation of TB infection may be preferred. For individuals or populations that are more likely to be infected and are at risk for progression to TB disease and adverse outcomes if TB disease develops, TPT is justified without testing.
People with HIV who are on ART benefit from TPT regardless of whether they test positive or negative for TB infection. WHO therefore recommends that testing for TB infection not be a requirement for initiating TPT among people with HIV and child contacts < 5 years, particularly in countries with a high TB incidence, given that the benefits of treatment (even without testing) clearly outweigh the risks (13). Furthermore, these tests are insensitive and may provide a false-negative result, particularly among immunocompromised hosts who are at a greater risk for severe forms of disease and death if they develop TB. The immune response to TB antigens varies, and tests for TB infection could be positive even after successful completion of TPT. Therefore, results of tests for TB infection should not be used to assess the efficacy of TPT. Overall, testing for TB infection should not be considered mandatory before starting TPT (especially when access to testing remains limited), given that the benefits of treatment (without testing) still outweigh the risks.
When national programmes recommend testing for TB infection before providing TPT, tests should be used only for at-risk groups (such as clinical risk groups, contacts of TB patients, prisoners and healthcare workers). Targeted testing allows identification, evaluation and treatment of people who are at high risk for TB infection or for developing TB disease when infected with M. tuberculosis. A positive test for TB infection among HIV-negative contacts or individuals in other clinical risk groups (patients initiating anti-TNF treatment, receiving dialysis, preparing for organ or haematological transplantation) may reassure clinicians and health-care workers that TB infection is likely, so that they can start TPT. A positive test of TB infection may motivate the person at risk to start and complete TPT.
4.3 Considerations for testing services for TB infection
The choice of a test for TB infection in a country might depend on the availability and affordability of the tests, the structure of the health system, the feasibility of implementation and infrastructure requirements. Considerations for testing for TB infection in general and for specific tests are described below. Details of how the tests work, test procedures and result interpretation can be found in the WHO operational handbook on tuberculosis. Module 3: diagnosis – tests for tuberculosis infection (53).
4.3.1 General requirements
- Define the target population for testing, and specify the choice of test in the national guidelines.
- Build the capacity of the health-care workers responsible for testing for TB infection, such as in administration of TST or TBST reading, collection and processing of blood specimens for IGRAs, specimen collection and transport.
- Develop SOPs for administration of TST or TBST, collection and processing of blood specimens for IGRA and interpretation of test results.
- Develop SOPs for appropriate follow-up after testing, including access to clinical evaluation, CXR and other TB investigations to decide on the eligibility of individuals for TPT.
- Develop job-aids to assist providers in informing the test recipient and responding to frequently asked questions on the utility and procedure of TST, TBST or IGRA.
- Develop tools for systematic recording and reporting of test results and linkage to care and treatment, such as the Prevent TB mobile application (54).
- Strengthen mechanisms for supportive supervision and monitoring of accurate implementation.
4.3.2 Programmatic implementation of TST and TBST
- Ensure the availability and supply of tuberculin or M. tuberculosis-specific antigens in the cold chain and of syringes, needles and other consumables.
- Train personnel in intradermal injection and in reading and interpreting tests, and provide ongoing capacity-building and supportive supervision to maintain skills. Ensure that staff can explain to the people they test what the result of the test implies in their case.
- Ensure standardized application of test procedures, mentoring, supervision and periodic standardized reliability testing for quality assurance.
- Develop and provide job-aids for health-care workers showing the correct techniques for TST and TBST administration and measurement of induration.
- Establish mechanisms to contact people who have been tested to return for the test reading within 48–72 h of administration of tuberculin or M. tuberculosis-specific antigens, or, alternatively, ensure test reading at the person’s residence.
- Provide funding for people to travel for testing or for health-care workers to administer and read test results.
- Develop and supply TST or TBST request forms, and update the HMIS to ensure documentation and reporting of TST and TBST results.
- As there is less experience in use of the new TBST, its safety is less well understood than that of TST. Therefore, surveillance of adverse events should be organized in countries that are initiating use of the new TBST.
4.3.3 Programmatic implementation of IGRA
- Develop the capacity of the laboratory system to conduct IGRA (phlebotomy, processing of blood specimens, incubation and ELISA reading). National programmes may collaborate with other, non TB-specific laboratories that have the capacity for drawing blood and ELISA testing or private institutions and laboratories through memoranda of understanding or free vouchers for individuals who require testing.
- Ensure the availability of trained laboratory technicians in laboratories in which IGRA tests are performed.
- Establish mechanisms to ensure rapid transport of blood specimens from peripheral centres to an IGRA testing laboratory (within 8–30 h) to allow incubation, depending on the type of IGRA.
- Ensure the functioning of laboratory equipment, and establish a mechanism for regular equipment maintenance for optimal functioning.
- Ensure a supply of appropriate reagents and testing tubes for IGRA, suitable for use at different altitudes (e.g. Johannesburg, South Africa, which is nearly 1700 m above sea level, requires different reagents or tubes from a place closer to sea level) (55).
- Ensure a supply of updated laboratory request forms and registers, and update laboratory information systems to document and report IGRA test results.
Fig. 6. Combined algorithm for screening and testing of individuals at risk before starting TB preventive treatment
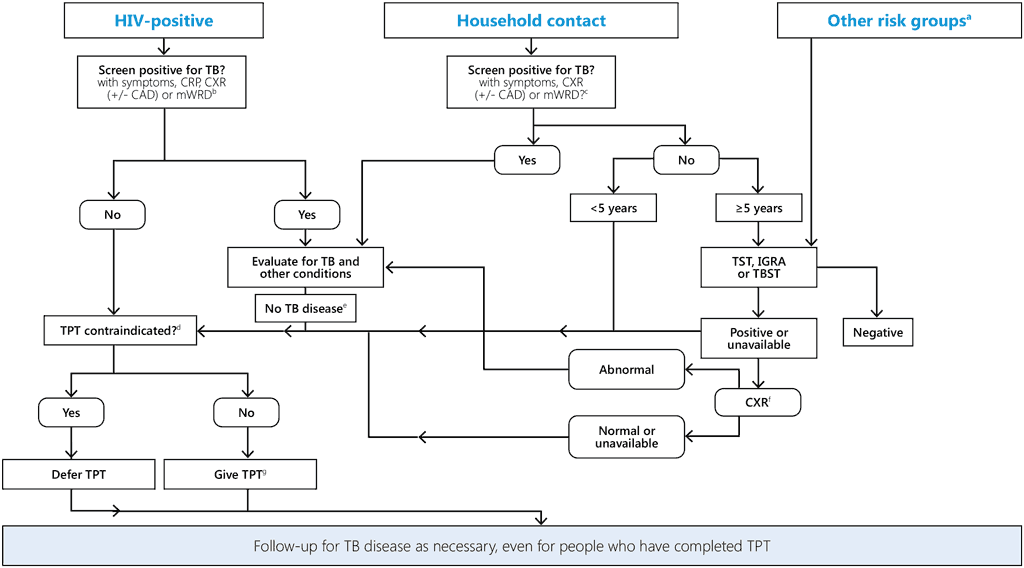
CAD, computer aided detection of TB; CRP, C-reactive protein; CXR, chest radiography; IGRA, interferon-γ release assay; mWRD, molecular WHO-recommended rapid diagnostic test; TB, tuberculosis; TBST, Mycobacterium tuberculosis antigen-based skin test; TPT, TB preventive treatment; TST, tuberculin skin test
a Including miners with silicosis, people on dialysis or anti-TNF agent treatment, preparation for transplantation or other risks in national guidelines. TB disease should be ruled out for people in this category.
b For children aged ≥ 10 years, a four-symptom screen is used (current cough or fever or weight loss or night sweats). For children aged < 10 years, consider their history of contact with TB or reported or confirmed weight loss or growth curve flattening or weight for age < –2 Z-scores. Asymptomatic infants aged < 1 year with HIV are given TPT only if they are household contacts of people with TB. For other screening options, see the latest WHO guidance (TB-KSP).
c Any one of cough or fever or night sweats or haemoptysis or weight loss or chest pain or shortness of breath or fatigue. In children, poor weight gain (plateau on growth chart), reduced playfulness or lethargy should also be included in symptom screening; cough may be absent. For other screening options see the latest WHO guidance (TB-KSP).
d Including acute or chronic hepatitis; peripheral neuropathy (if isoniazid is used); regular and heavy alcohol consumption. Pregnancy or a previous history of TB are not contraindications. The person is counselled about the benefits and potential risks of TPT.
e In household contacts aged ≥ 5 years, TST, IGRA or TBST is recommended before consideration of TPT.
f CXR is required only if it was not conducted at a previous step.
 تعليق
تعليق