Book traversal links for Annex 2. Messages for different stakeholders
Messages for ministries of health
- National governments renewed their commitments to fight TB at the second UN High-level Meeting on 22 September 2023 (1) and:
- pledged to accelerate progress towards timely, quality universal access to tuberculosis services in both high and low burden countries, as outlined in the End TB Strategy, such that, by 2027, at least 90 per cent of people at high-risk of developing tuberculosis are provided with preventive treatment, which translates to providing up to approximately 45 million people with TB preventive treatment, including approximately 30 million household contacts of people with tuberculosis, including children and approximately 15 million people living with HIV, with the vision of reaching more people, including those who live in remote geographical regions or in areas difficult to access, taking into account World Health Organization guidance.
- In May 2014, national governments endorsed a World Health Assembly resolution for an End TB Strategy and its targets to end the global TB epidemic (2), with targets to reduce TB deaths by 95% and to reduce the number of new cases by 90% between 2015 and 2035. The strategy includes a target to provide TPT to 90% of those eligible by 2025.
- TPT is a proven, effective intervention for averting the development of TB disease; it reduces the risk to 60–90% of that of people who do not receive TPT (3).
- TPT given to people at the highest risk of progressing from TB infection to disease remains a critical intervention to end TB worldwide. TPT is part of a larger group of actions to eradicate poverty – from screening for TB disease, TB infection control, prevention and care of HIV, management of other comorbidities and health risks, better access to universal health care and social protection.
- Large numbers of deaths due to TB could have been avoided if TPT had been made available worldwide after recommendation for its programmatic use in 2008 (4). Urgent steps for nationwide implementation should therefore be taken to prevent massive suffering, catastrophic costs and deaths. In countries in which such programmes start now, achievement of the End TB targets will be accelerated.
- PMTPT is a key element of TB elimination in all settings and should be undertaken aggressively, particularly in settings with a low TB incidence.
- Shorter, rifamycin-based TPT (4R, 3HR, 3HP, 1HP) provides alternative options to IPT, which has been the main approach up until now. A shorter TPT regimen is more likely to be completed, as it is more tolerable and easier to manage programmatically; it may therefore have greater potential to save lives. Demand for access to TPT should be increased by raising awareness among people at risk for TB and TB-affected communities. National programmes should be mindful that they are accountable for delivering TPT.
- Access to rapid tests and investigations to diagnose TB disease and TB infection (such as Xpert MTB/Rif, CXR, urinary LAM, TST, TBST or IGRA) should be increased by investments in infrastructure, human resources and logistics for nationwide scaling up of TPT.
- Mechanisms and investment are required for building the capacity of nurses and other healthcare workers in counselling people with HIV and people with TB and their families and contacts, to understand TPT, initiate TPT, follow-up treatment, identify and manage adverse events and signs of toxicity, and decide when to stop TPT.
- Investment in strengthening systematic recording and reporting for PMTPT with digital tools would improve monitoring of progress in programme management and resource allocation.
- Priority groups for TPT include household and other close contacts of people with TB, people with HIV and people with other immunodeficiency or predisposing clinical conditions (such as silicosis, dialysis, organ or blood transplant). National programmes may consider including TPT and TB screening activities for at-risk populations.
Messages for health-care workers
- TPT saves lives, prevents transmission of infection and illness and averts suffering due to TB. Strong proof comes is provided by the TEMPRANO trial, in which use of IPT for people with HIV in Côte d’Ivoire. A 37% reduction in mortality was observed among those who received IPT, independently of whether they were also on ART, and people on both IPT and ART had the greatest protection against severe disease and death (5).
- Currently recommended TPT regimens offer durable protection after one course in people with HIV, HIV-negative contacts and other at-risk populations. The protection lasts for 6–19 years.
- Some TPT regimens comprise two TB drugs – isoniazid and rifapentine or rifampicin – and are taken for only 1 or 3 months. They are as effective as IPT in preventing progression to TB disease and are easier to complete. While they may cost more in the short term, they provide more cost-effective protection, as people on shorter drug regimens are up to three times more likely to complete their course of TPT than those on longer regimens, leading to better outcomes and more lives saved.
- Table A2.1 summarizes the steps to be considered by health-care workers in initiating TPT. Counselling of people at risk and their families enables an informed decision on whether to accept TPT and adhere to the treatment schedule. People on TPT should be educated about the signs and symptoms of serious adverse events, such as drug-induced hepatitis, and encouraged to report adverse events promptly.
- Explain to an individual that a course of medical treatment lasting weeks to several months is needed even if she or he is not sick. It is also important to support and ensure adherence to completion of the full course of TPT.
- There is no clear evidence that PMTPT increases resistance to TB drugs. Nevertheless, all efforts should be made to rule out TB disease with recommended procedures. If a screening test is negative, the likelihood of TB disease is minimal. In such cases, withholding TPT is a missed opportunity to protect individuals and communities from avoidable disease and death and could hence be viewed as unethical.
- Concern about harming otherwise healthy individuals must be addressed. A very small proportion of people on TPT develop adverse events, most of which are self-limiting and reversible. The shorter rifamycin-based regimens are safer than others. The availability of alternative options can help to minimize the risk.
- All people prescribed TPT should be informed clearly of the schedule of treatment, possible adverse events (“side-effects”) and health alerts and to contact their health-care provider or stop TPT.
- Systematic recording and reporting are important both to inform individual care and to monitor indicators of programme performance.
- With appropriate training, nurses and other front-line health-care workers can undertake most of the clinical duties required in PMTPT. They include deciding on testing for TB infection and TB disease, interpretation of results, establishing eligibility for TPT, starting TPT and monitoring adherence, and deciding whether TPT should be suspended or changed (e.g. in the case of adverse events) or re-started (e.g. after an interruption by the person on treatment). There is usually no need to solicit the opinion of a medical doctor or a specialist for such decisions, although this should be available if necessary.
Table A2.1. Steps in starting TPT
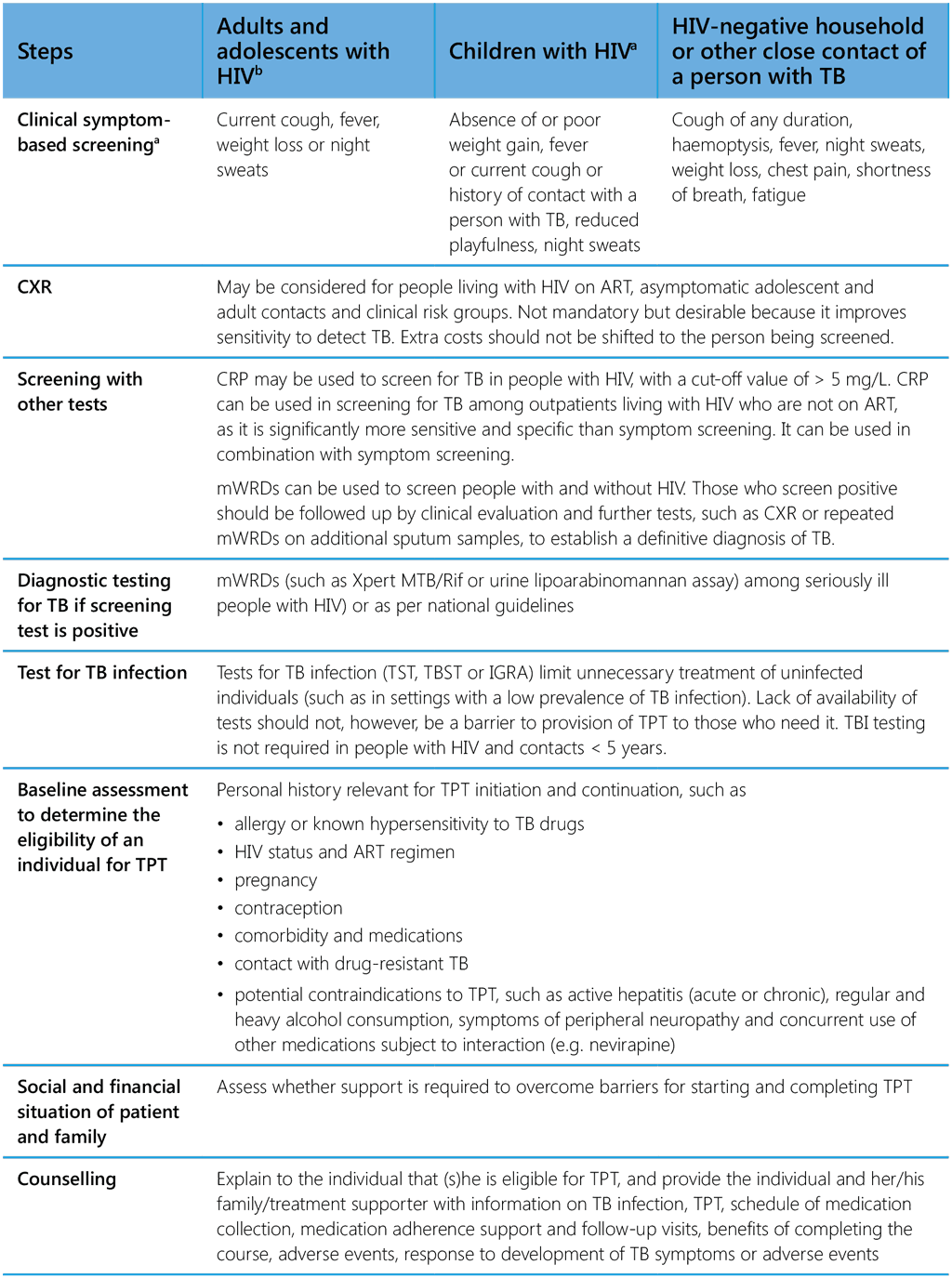
ART, antiretroviral therapy; CRP, C-reactive protein; CXR, chest radiography; HIV, human immunodeficiency virus; IGRA, interferon-γ release assay; mWRDs, molecular WHO-recommended rapid diagnostic tests; TB, tuberculosis; TBI, TB infection; TBST, Mycobacterium tuberculosis antigen-based skin test; TPT, TB preventive treatment; TST, tuberculin skin test
a Screening of children and pregnant or breastfeeding women can be integrated into other care, such as for maternal and child health, vaccination, well-baby clinics, nutrition clinics.
b Among people with HIV, all the above steps should be included if differentiated HIV service delivery models are used. Screening and TPT should be an integral part of the care package for people with HIV.
Messages for people with HIV and other people offered TPT
- You (your family members) do not have TB disease. You (your family members) may have an infection that could become TB disease. TB is a serious disease, could threaten your life and could spread to your family, neighbours and co-workers.
- Your doctor has determined that you would benefit from TPT (for you or your family members) although you and your family are currently healthy. TPT can reduce your risk of getting TB by 60–90%. In most individuals, TPT will not cause any discomfort or adverse events (“side-effects”); however, if adverse events occur, your caregiver will visit you regularly and provide care. Your healthcare provider will inform you of the common adverse events of the TPT you are offered. You are free to take TPT, opt out or stop it after starting.
- The protection offered by TPT is optimal only when the prescribed course of TPT is completed as expected. If you decide to take TPT, please remember to take it as indicated by your healthcare provider.
- If you (or your family members) notice any adverse event, consult your health-care worker as soon as possible. If danger signs are noted (such as signs of jaundice – yellowing of the skin and whites of the eyes), stop TPT, and seek care and support in a health facility.
- If you (or your family members) are on rifamycin-based TPT and wish to avoid pregnancy, please note that rifapentine (and other rifamycins) decrease the effectiveness of hormonal contraceptives (6). You (your family members) should consider using a different or barrier form of contraception when taking rifapentine or a rifampicin-based TPT.
- Parents and legal guardians: giving your children TPT will protect them from TB, which can be difficult to diagnose and could have long-lasting effects. Child-friendly medicines that dissolve in water and have a nice taste are now available to make it easier for your child to take treatment regularly.
Messages for communities
- TB is a contagious disease that is transmitted through air when a person with infectious TB coughs. TB is associated with considerable morbidity and mortality, even when treated. Even when people with TB complete treatment, some are left with considerable damage to their lungs or to other organs, which can seriously affect their quality of life.
- TB is preventable, and prevention is much better than cure. A number of options are available to prevent TB and to reduce the burden of TB in the community. They include early detection and treatment, BCG vaccination of infants and providing TPT to individuals who are currently well but have been exposed to TB or are at a high risk of developing TB disease.
- To reduce the number of individuals who develop TB each year, countries have committed themselves to providing TPT to people who have been exposed or already have TB infection in their bodies even if it has not yet progressed to TB disease, such as in people with HIV, and family members of people with TB, including children. Providing treatment to these individuals will prevent them from developing TB disease and result in a healthier community.
- TB infection is extremely common. Community members who require TPT are not sick, are not coughing and are at no risk of transmitting TB. TPT is prescribed to minimize an individual’s future risk of developing TB disease. This also protects the community, because TB is a contagious disease.
- The drugs used for TPT are generally very safe. Shorter TPT regimens with a combination of two TB drugs – isoniazid with rifapentine or rifampicin – are now available, which are effective in preventing progression to TB disease. These TPT regimens have fewer side-effects and are easier for people to take. It may, however, be difficult for people with TB infection who do not show symptoms to understand that they must take a medication to treat infection. Although treatment of TB disease lasts ≥ 6 months, shorter TPT regimens that can be completed in 4–12 weeks are now available. All TPT must be completed as prescribed in order to be effective.
- Individuals may find it difficult to complete a full course of TPT. Community health workers, affected communities, TB survivors, civil society organizations and nongovernmental organizations can support people who are taking TPT to finish it.
- By keeping adults free from TB, children will be able to avoid being exposed to TB and live healthier lives as they grow up. Keeping people with HIV free from TB reduces their suffering and help them live healthier, longer TB-free lives.
- People with HIV who are responding well to ART may still contract TB. Their TB infection may go unnoticed and untreated for long, until it is too late. Taking TPT will ensure that people with HIV will be protected from TB disease. Not taking TPT is a missed opportunity to prevent unnecessary sickness or even death.
- Most children who become infected with TB have been infected by an adult – whether a parent or another person in the household. They are also at higher risk of developing TB in the following years and would benefit from TPT. It is important that when someone in the family has been identified as having TB disease, family members, including children, are evaluated and encouraged to take TPT.
- Demand should be created by sharing information with communities about accessing TPT and by promoting TPT among people who should be protected from TB infection and disease.
- The HIV community, people affected by TB, TB survivors, civil society organizations working with children and civil society organizations and NGOs working on TB could advocate strongly for TPT. Their role is important for screening household and community contacts for symptoms, encouraging and referring people to access TPT, lobbying and advocating local and national health ministries for allocation of resources and increasing demand for TPT in their countries and localities.
An example of a community flyer promoting TPT is available in Annex 1 of the first edition of this operational handbook (pages 99–102) (7)
References
- The second United Nations high-level meeting on TB: new global pledge to end the TB epidemic. declaration on TB. Geneva: World Health Organization; 2023 (https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023/featured-topics/un-declaration-on-tb).
- The End TB Strategy. Geneva: World Health Organization; 2024 (https://www.who.int/teams/global-tuberculosis-programme/the-end-tb-strategy).
- Lobue P, Menzies D. Treatment of latent tuberculosis infection: An update. Respirology. 2010;15(4):603–22. doi:10.1111/j.1440–1843.2010.01751.x.
- Chaisson RE, Golub JE. Preventing tuberculosis in people with HIV – no more excuses. Lancet Glob Health. 2017;5(11):e1048–9. doi:10.1016/S2214–109X(17)30390-X.
- Badje A, Moh R, Gabillard D, Guéhi C, Kabran M, Ntakpé JB et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob Health. 2017;5(11):e1080–9. doi:10.1016/S2214–109X(17)30372–8.
- Borisov AS, Bamrah Morris S, Njie GJ, Winston CA, Burton D, Goldberg S et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. Morb Mortal Wkly Rep. 2018;67(25):723–6. doi:10.15585/mmwr.mm6725a5.
- WHO operational handbook on tuberculosis; module 1; prevention; tuberculosis preventive treatment. Geneva: World Health Organization; 2020 (https://apps.who.int/iris/bitstream/handle/10665/331525/9789240002906-eng.pdf).
 تعليق
تعليق