Book traversal links for Annex. Summary of changes in policy on DS-TB treatment since 2010 and mapping of recommendations in consolidated DS-TB guidelines
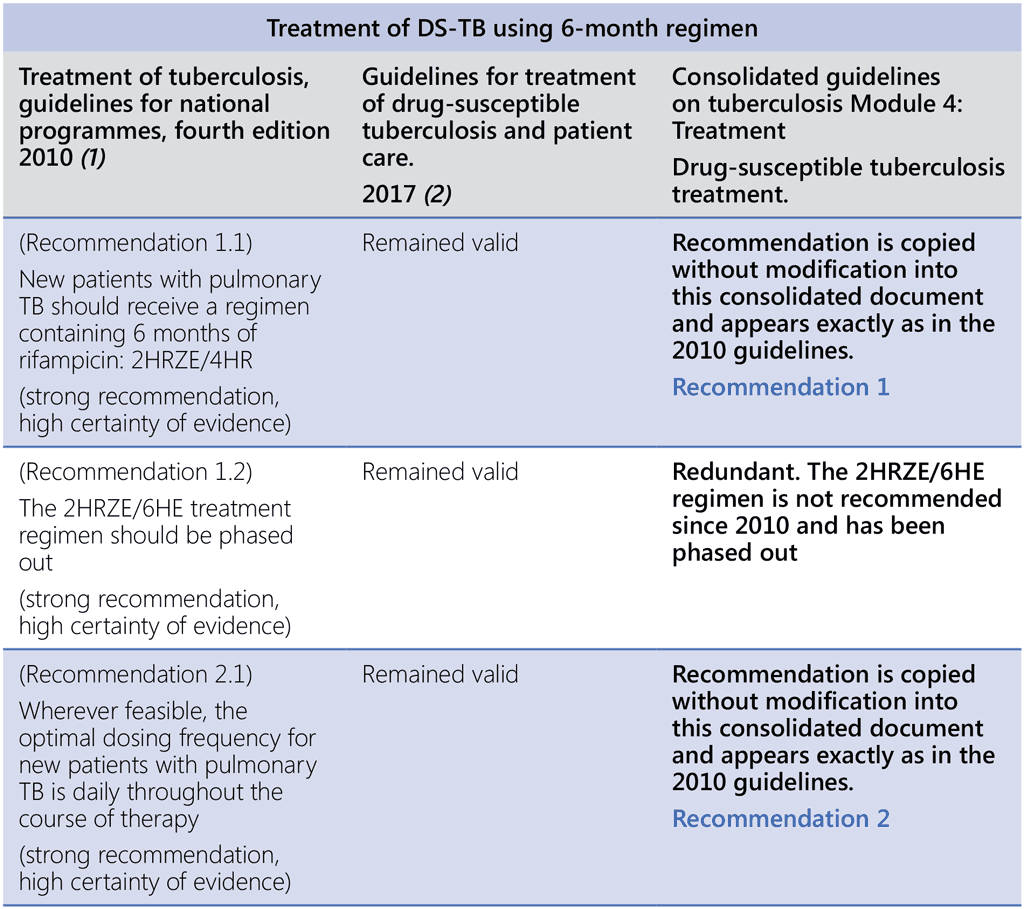
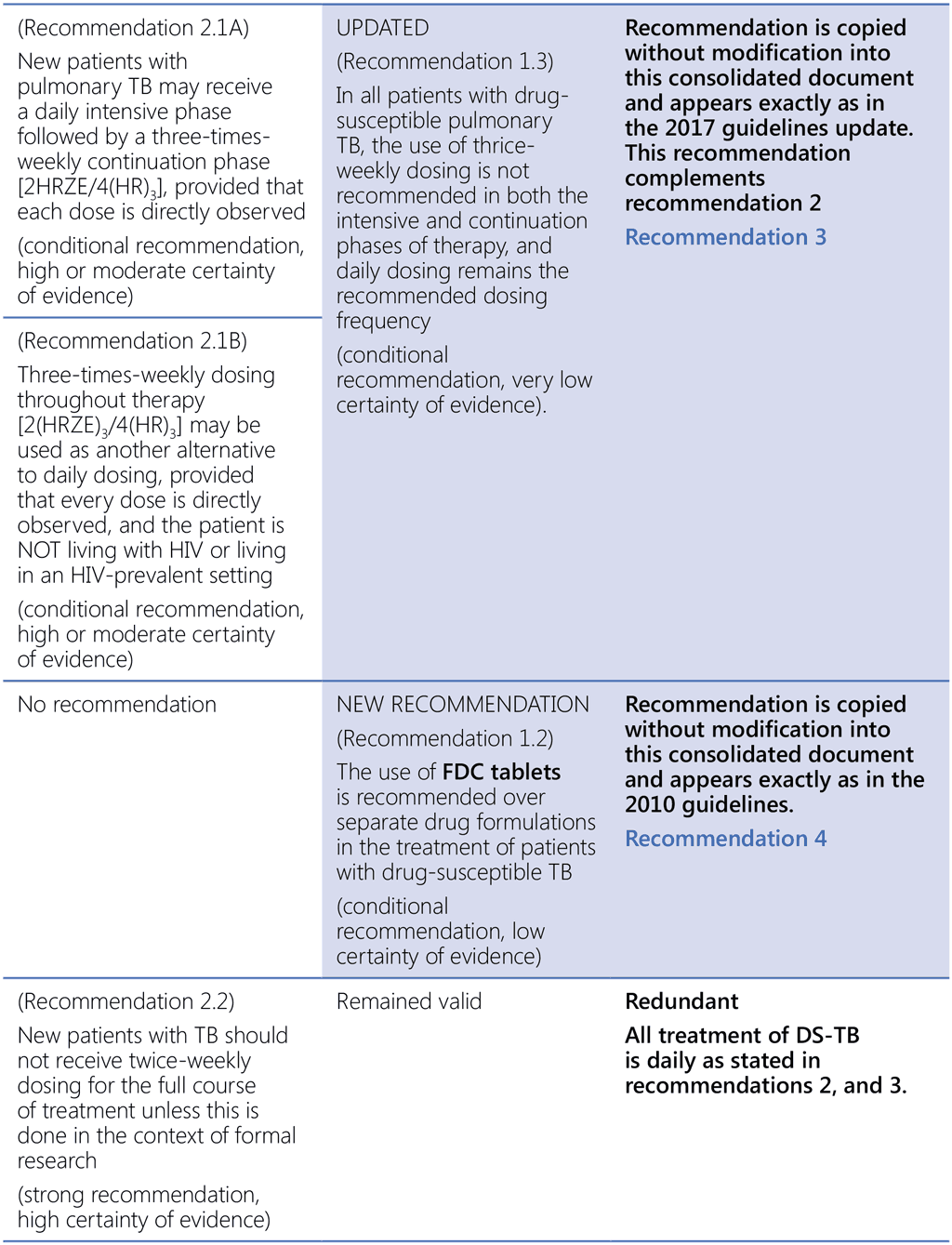
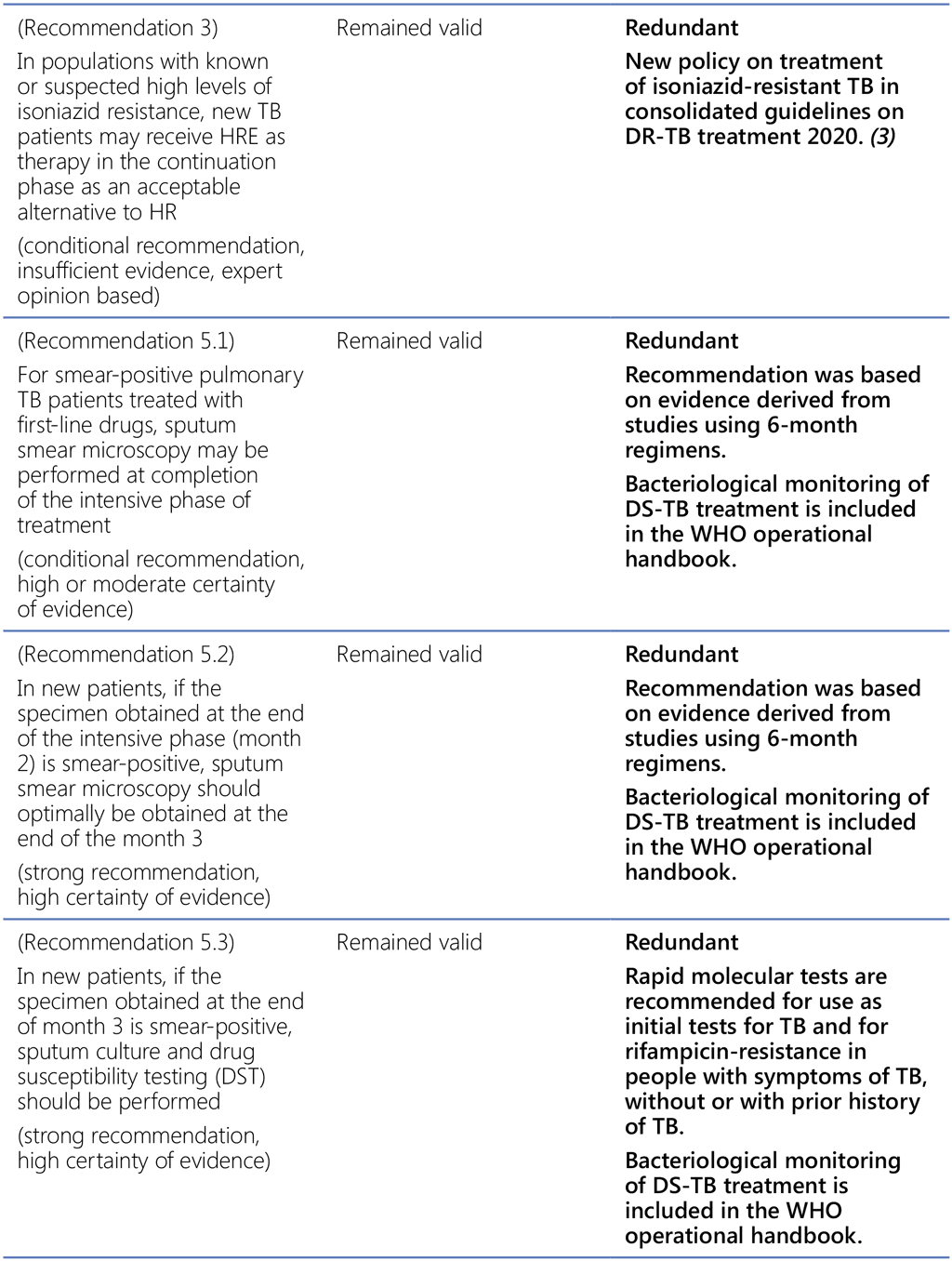
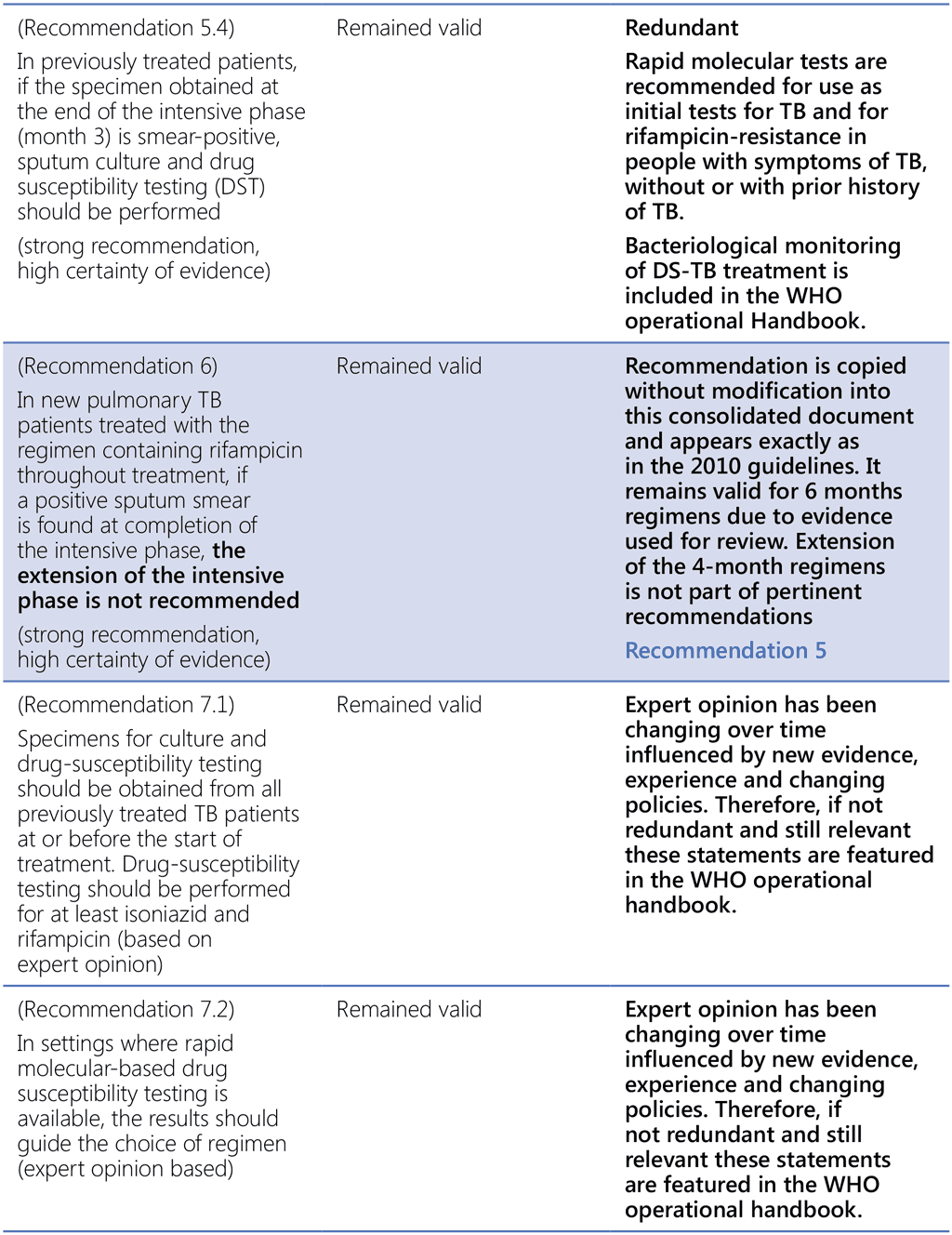






References to WHO guidance:
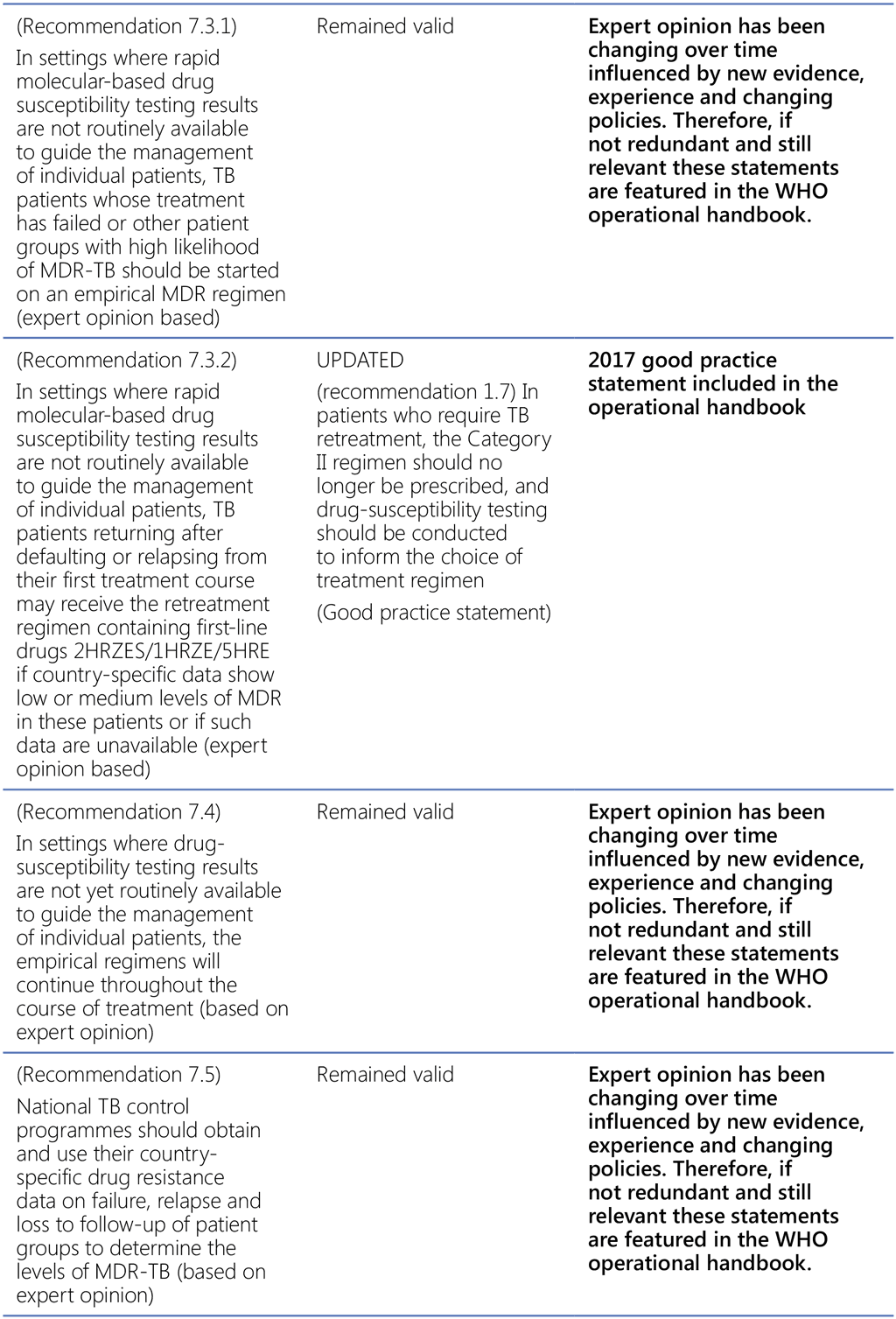
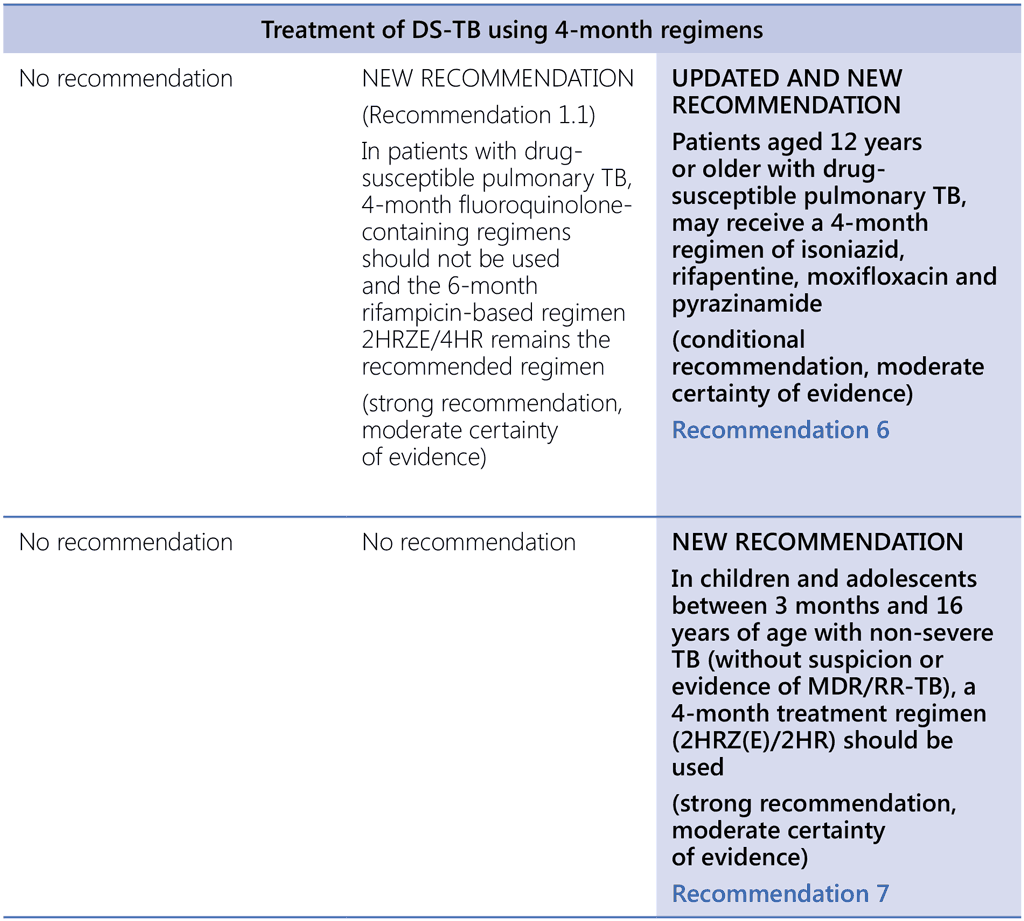
- Treatment of tuberculosis, guidelines for national programmes, fourth edition. Geneva: World Health Organization; 2010 (https://www.who.int/publications-detail-redirect/9789241547833, accessed 3 March 2022).
- Guidelines for treatment of drug-susceptible tuberculosis and patient care. Geneva: World Health Organization; 2017 (https://apps.who.int/iris/bitstream/handle/10665/255052/9789241550000-eng.pdf, accessed 3 March 2022).
- WHO Consolidated guidelines on tuberculosis, Module 4: Treatment – Drug-resistant tuberculosis treatment. Geneva: World Health Organization; 2020 (https://www.who.int/publications-detail-redirect/9789240007048, accessed 3 March 2022).
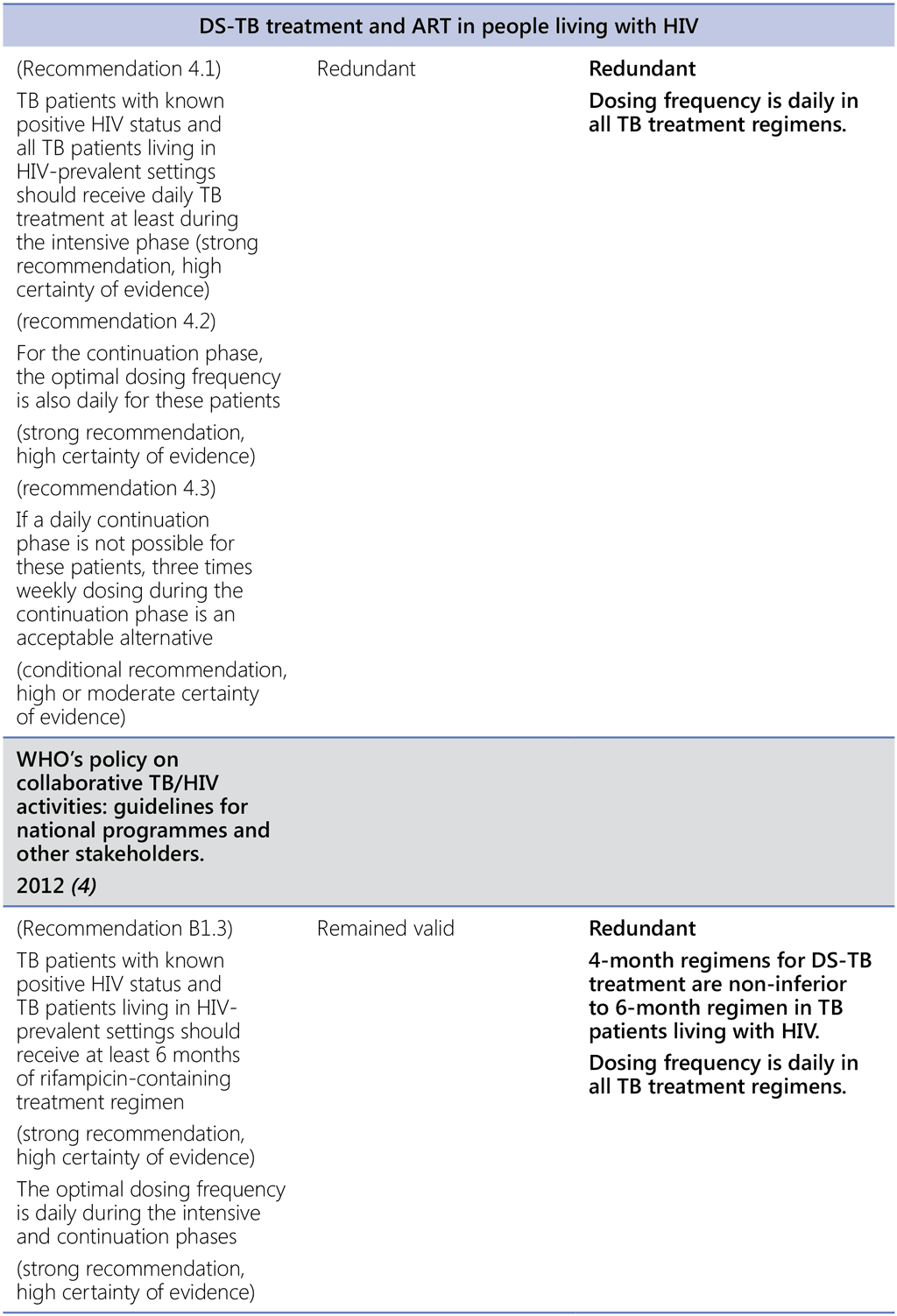
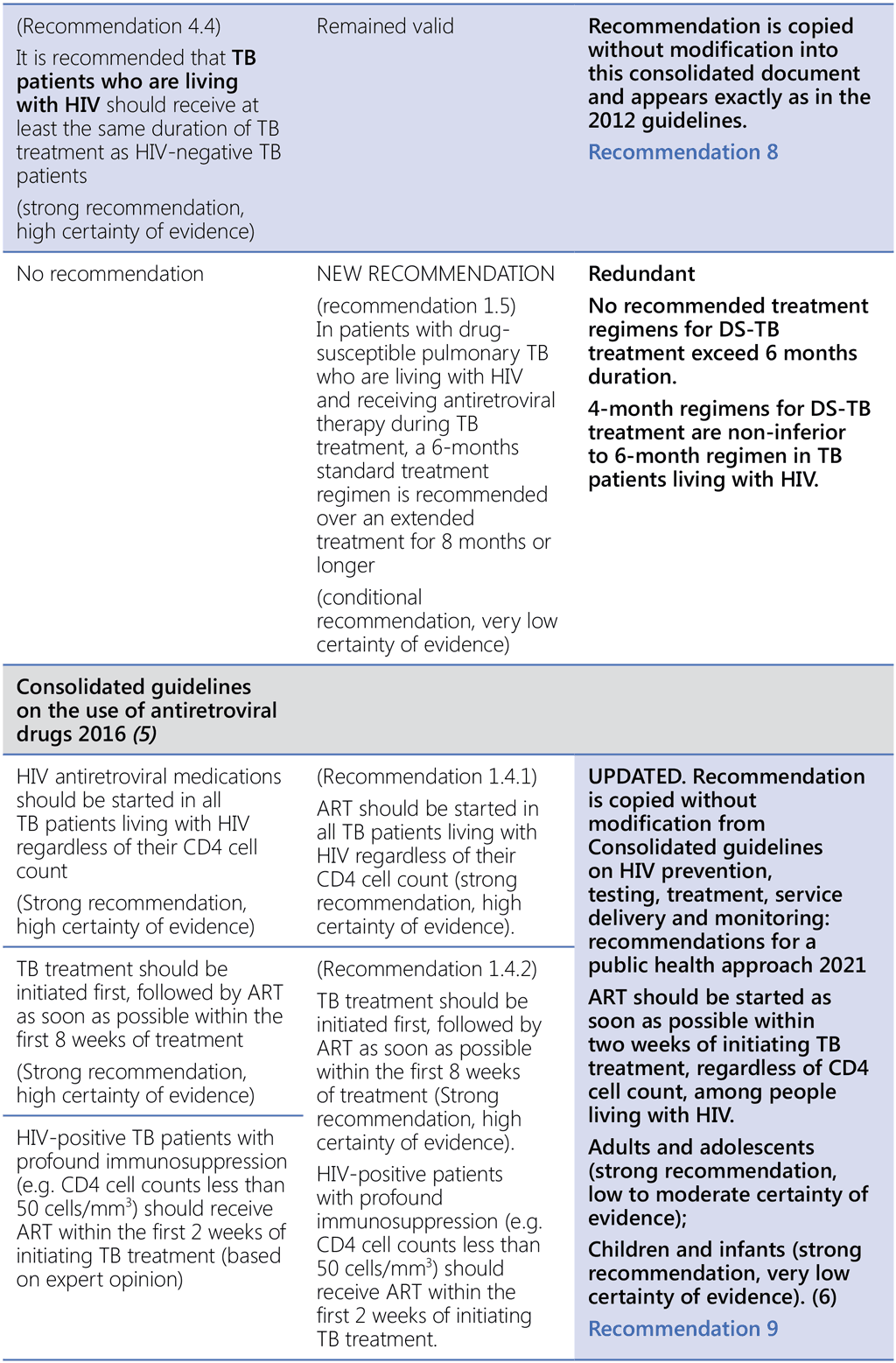
- WHO policy on collaborative TB/HIV activities: guidelines for national programmes and other stakeholders. Geneva: World Health Organization; 2012 (https://apps.who.int/iris/handle/10665/44789, accessed 3 March 2022).
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, second edition. Geneva: World Health Organization; 2016 (https://apps.who.int/iris/handle/10665/208825, accessed 3 March 2022).
- Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva: World Health Organization; 2021 (https://www.who.int/publications-detail-redirect/9789240031593, accessed 3 March 2022).
- Guidelines for the programmatic management of drug-resistant tuberculosis. Geneva: World Health Organization; 2011 (https://www.who.int/publications-detail-redirect/9789241501583, accessed 3 March 2022).
- Guidance for national tuberculosis programmes on the management of tuberculosis in children. Geneva: World Health Organization; 2014 (https://www.who.int/publications-detail-redirect/9789241548748, accessed 3 March 2022).
 تعليق
تعليق