Book traversal links for 5.2 Justification and evidence
The recommendation in this section addresses the following PICO question:
PICO question (MDR/RR-TB, 2018). In patients with MDR/RR-TB treated with longer or shorter regimens composed in accordance with WHO guidelines, is monitoring using monthly cultures, in addition to smear microscopy, more likely to detect non-response to treatment?
Previous studies have indicated that monthly culture is the optimum strategy to detect non-response as early as possible and was conditionally recommended by WHO in 2011 as the preferred approach (8, 105, 106). The findings of the evidence review and analysis performed for this question are expected to influence the continued validity, in its present form, of the 2011 WHO recommendation (8). Since then, significant changes in MDR-TB treatment practices have taken place on a large scale globally, such as the wider use of later-generation fluoroquinolones, bedaquiline and linezolid; a tendency towards an intensive phase of longer duration; and the widespread use of the shorter regimen, which could influence the speed and durability of culture conversion during the continuation phase, when this PICO question is of greatest relevance.
Achieving sustained bacteriological conversion from positive to negative is widely used to assess response to treatment in both drug-susceptible TB and DR-TB. Culture is a more sensitive test for bacteriological confirmation of TB than direct microscopy of sputum and other biological specimens. Culture also facilitates phenotypic testing for DST, a critical consideration in TB diagnostics. However, performing culture requires considerable logistical organization and a well-equipped laboratory to limit cross-contamination, ensure proper bacterial growth and match other quality standards. Apart from the resource requirements, culture results become available after a significant delay of weeks or months, contrasting markedly with the relative immediacy of the result of direct microscopy (although microscopy cannot confirm mycobacterial viability). Although molecular techniques can now provide a rapid and reliable diagnosis, they cannot replace culture or microscopy for the monitoring of bacteriological status during treatment.
The evidence used to explore the added value of culture over sputum smear microscopy alone, and the optimal frequency of monitoring, was obtained from a subset of the IPD reported to WHO by South Africa for the 2018 update. These observational data from South Africa comprised 26 522 patients overall. Of these, 22 760 records were excluded from the dataset for the following reasons: 11 236 had a treatment outcome of death or loss to follow-up; 698 had a successful treatment outcome but had received less than 17.5 months of treatment; 1 357 had fewer than six culture samples recorded; 1 632 had no baseline culture recorded; 2 502 were baseline culture negative; 2 920 were smear negative at baseline or had a missing smear at baseline; and 2 415 had insufficient smear data to match the culture data. This left 3 762 MDR/RR-TB patients (with 1.8% being children; i.e. aged <15 years) treated with longer MDR-TB regimens between 2010 and 2015, who had both monthly smear and culture data throughout treatment to address PICO question 11 (MDR/RR-TB, 2018). About 60% of these patients were People with HIV. The analysis focused on whether monthly culture versus monthly smear microscopy or culture every 2 months is needed to not miss treatment failure in MDR/RR-TB patients on treatment. The odds of treatment failure in patients who do not convert at 6 months or later was also discussed (see Section 5.4 and Table 5.1). The data could not address the outcome on acquisition (amplification) of additional drug resistance, nor could it assess directly whether the frequency of culture or smear microscopy had an identical effect on failure in patients on the 9–12-month shorter MDR-TB regimen, as envisaged in the original PICO question 11 (MDR/RR-TB, 2018). Based on an assessment of the certainty of the evidence, carried out using predefined criteria and documented in GRADEpro, the test accuracy certainty of the evidence was rated as moderate.
The IPD meta-analysis compared the performance of the two methods in terms of sensitivity and specificity, and culture testing once a month compared with once every 2 months (to assess the minimum frequency of testing needed to not unnecessarily delay any revision of the treatment). The focus of the analysis was to compare how the two tests performed in terms of predicting treatment failure or relapse.
The main findings of the analysis were that monthly culture had a higher sensitivity than monthly smear microscopy (0.93 vs 0.51) but slightly lower specificity (0.97 vs 0.99). Likewise, the sensitivity of culture done every month was much higher than once every 2 months (0.93 vs 0.73) but had a slightly lower specificity (0.97 vs 0.98). Monthly culture increased the number of patients detected with a true positive bacteriological result by 13 per 1 000 patients and reduced false negative results by 13 per 1 000 patients when compared with sputum smear microscopy alone. In contrast, monthly culture was estimated to lead to 17 per 1 000 fewer true negative results and 17 per 1 000 more false positive results for treatment failure, implying that treatment may be prolonged in the case of false positivity or missed true negativity. The added inconvenience to the patient and programme is considered relatively small, given that taking sputum and many other biological specimens is usually non-invasive and routine practice in many programmes. In a setting where testing is repeated at monthly intervals, a single false positive test result is unlikely to prove harmful to the patient because treatment decisions usually rely upon at least two consecutive positive results (to denote prolonged positivity or reversion) and the effect of one spurious result would last only until the test repeated 1 month later is reported.
The crude odds of treatment failure increased steadily with each additional month without bacteriological conversion, from 3.6 at the end of the first month to 45 at the eighth month when using culture (Table 5.1). However, no discrete cut-off point (to serve as a reliable marker of a failing regimen) could be discerned at which the odds of failure increased sharply when monitoring with either sputum smear microscopy or culture. The threshold for when to change treatment thus depends on the clinician’s desire to minimize the risk of failure and, in particular, to limit the risk of prolonging a failing regimen.
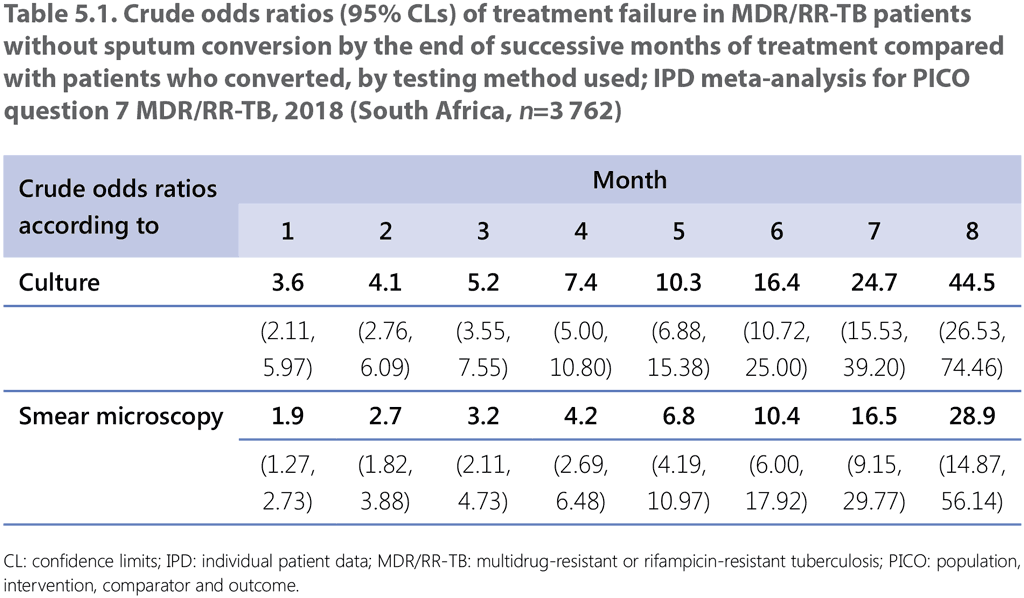
There was moderate certainty in the estimates of test accuracy and the GDG considered that, under normal conditions, culture would always be a more sensitive test of positive bacterial status than sputum smear microscopy. However, the overall quality of the evidence was judged to be low. The effects observed may vary in patients or populations with a profile markedly different from the one included in the analysis, such as low HIV-prevalence settings, children, patients with extrapulmonary forms of disease or those treated with the shorter MDR-TB regimen. The 3 762 patients included in the analysis had similar clinical characteristics to the 22 760 individuals excluded, although they were slightly less likely to be HIV coinfected, have a history of previous treatment or have second-line drug resistance. Conversely, the rate of failure in those included in the analysis was only 3% compared with 12.7% of those excluded from the analysis.
 تعليق
تعليق