Book traversal links for 4.5.2 Monitoring safety
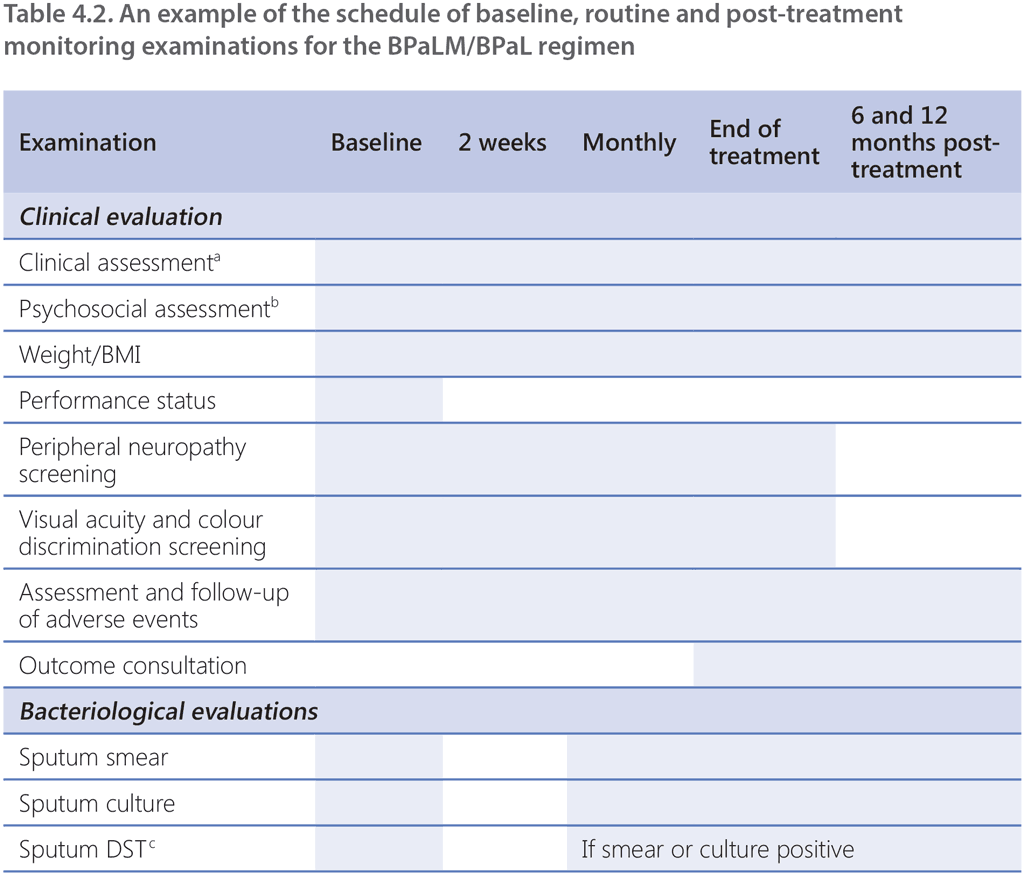
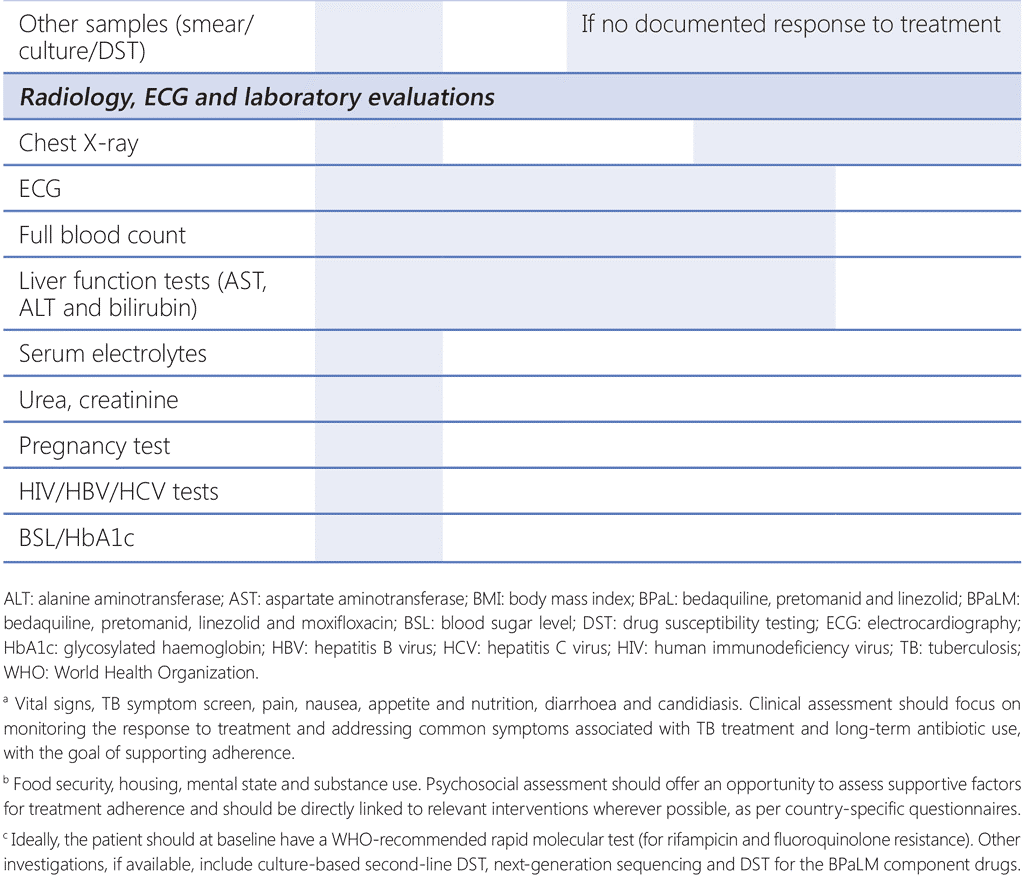
As with all TB programmes, active surveillance for adverse events is critical to ensure safety and minimize short-term and long-term morbidity of patients. A schedule for monitoring examinations should be established and applied to all patients receiving treatment with the BPaLM/BPaL regimen. Patients should undergo appropriate evaluation at the beginning of treatment (baseline), and during and after treatment. Common adverse events for each drug used in the BPaLM/BPaL regimen can be found in Web Annex 1, and a schedule of recommended investigations at baseline and during follow-up is given in Table 4.2. The monitoring schedule should consider the following:
- laboratory and ECG monitoring should be continued at monthly intervals for the duration of treatment (i.e. 9 months in the case of treatment prolongation);
- in the case of electrolyte disturbances, haematologic or ECG abnormalities, more frequent monitoring may be performed; and
- more frequent monitoring may be advisable in specific situations; for example, in older people, People with HIV, those affected by hepatitis (caused by hepatitis B virus [HBV] or hepatitis C virus [HCV]), those with diabetes mellitus, those with moderate to severe hepatic or renal impairment, or those with baseline anaemia or visual disturbances (e.g. glaucoma, cataract or colour blindness).
The WHO framework for aDSM needs to be applied to patients on any type of MDR-TB regimen, to ensure appropriate action and an acceptable level of monitoring for and prompt response to adverse events (Web Annex 3); the framework should be applied alongside monitoring for treatment outcomes, including early monitoring for treatment failure. Additional evidence on adverse events will be important to build the evidence base on the safety of the BPaLM/BPaL regimen in varied settings. Monitoring of changes in dosing and duration of linezolid can also inform the future evidence base on the wider use of the BPaLM/BPaL regimen and the tolerability of linezolid in this regimen.
The NTP should thus actively monitor drug safety to ensure proper patient care, to report any adverse events to the responsible drug safety authority in the country, and to inform national and global policy.
 تعليق
تعليق