Book traversal links for 3.1.1 Interpretation of TST – criteria for a positive TST
The sensitivity of TST based on different size criteria of induration was established among people who had been treated for and had recovered from microbiologically confirmed TB (34). Of these, 98% had a reaction of 5 mm or more to TST, 90% had a reaction of 10 mm or more but only 60% had a reaction of 15 mm or more. On the other hand, reactions to TST due to BCG or nontuberculous mycobacteria are usually smaller than TST reactions due to true infection with M. tuberculosis (11, 12). Therefore, setting higher cut-off points for a positive TST will improve specificity, but will reduce sensitivity, especially if a cut-off point of 15 mm is used (35). Hence, most clinical guidelines suggest using different size criteria for different populations, epidemiological situations and comorbidities (Table 3.1). If risk of TB disease is high, then sensitivity should be maximized; however, if risk of TB disease is low (or risk of adverse events from TPT is high), then cut-off points should be adjusted upwards to maximize specificity. In groups for which systematic TB infection testing and TPT are not recommended, an incidental finding of positive TST beyond the thresholds in Table 3.1 should prompt further queries about other risk factors that may warrant TPT (e.g. contact with an infectious TB patient). Otherwise, these individuals may be advised to return to health care immediately if symptoms associated with TB emerge.
Table 3.1. Criteria for size of TST induration by population (based on studies using PPDS or bioequivalent) (36)
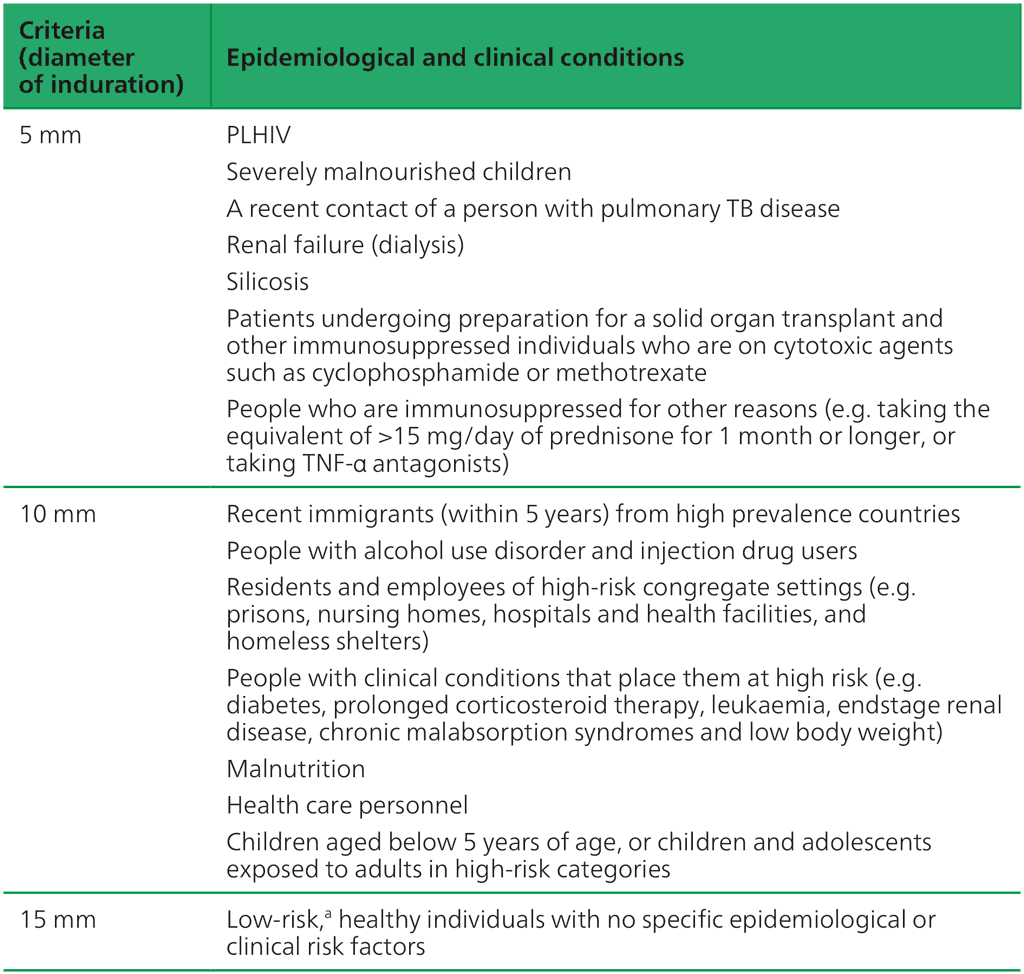
People with HIV: people living with human immunodeficiency virus; PPDS: standard purified protein derivative; TB: tuberculosis; TNF: tumour necrosis factor; TST: tuberculin skin test; WHO: World Health Organization.
ᵃ Current WHO guidelines do not recommend testing of low-risk individuals. An incidental finding would require further evaluation for other risks and close follow-up.
 تعليق
تعليق