Book traversal links for 3.3 Algorithms for informing the decision to treat TB infection or TB disease among people living with HIV
For people living with HIV, screening and diagnostic algorithms should serve both to include TB disease to identify those requiring treatment and to exclude TB disease to determine eligibility for TPT. Lack of access to any of the tools described here should not be a barrier to TB screening or ruling out TB to allow initiation of TPT. Here we present five practical algorithms that combine WHO-recommended TB screening tools (W4SS, CRP, CXR and mWRD) with diagnostic tools (LF-LAM and mWRD) for people living with HIV. The algorithms may be adapted to the local context according to factors including, but not limited to, epidemiology, feasibility, the level of the health facility where they are being implemented, resources and equity concerns.
Fig. 3.1 outlines the suggested placement of screening tools and initial diagnostic tests at the community level, the primary health care level and secondary care. Programmes should ensure that health facilities are connected to reliable and rapid sample transportation networks to facilitate collection and delivery of samples, and to ensure an efficient turnaround time and communication of results to the clinician. TB and HIV programmes should also collaborate to ensure that different cadres of healthcare workers, including lay health workers, are trained to collect samples for screening and diagnosis of TB, the use of the relevant tools and recording and reporting.
Fig. 3.1. Placement of screening tools and initial diagnostic tests
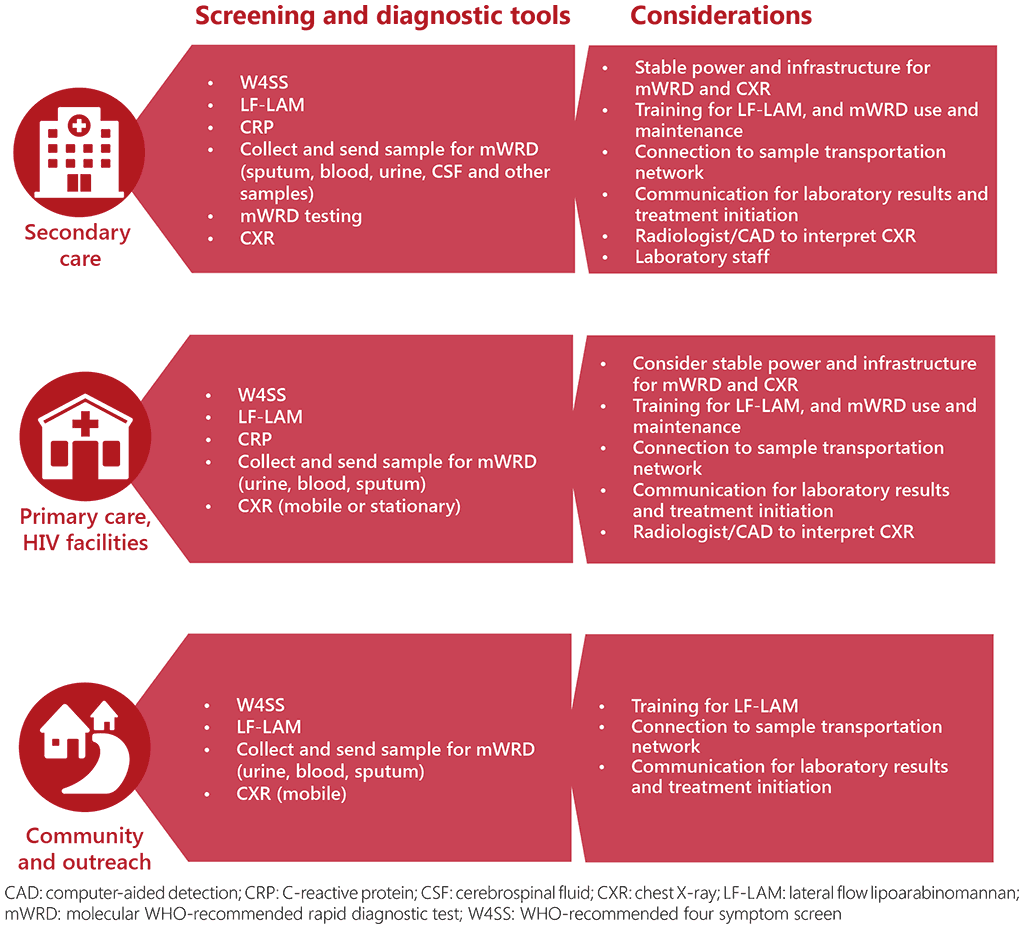
The WHO operational handbook on tuberculosis. Module 2: Screening: systematic screening for tuberculosis disease (76) details key considerations for the choice of screening and diagnostic algorithms. These include: the specific objectives of screening; the accuracy and yield of screening and diagnostic tests; the profile of prioritized risk groups; the TB prevalence in the risk groups; the costs, availability and feasibility of different tests; and the ability to engage the population to be screened. In practice, when implementing an algorithm, its performance will also be influenced by external factors such as connection to and reliability of the sample transportation network, loss to follow-up between screening tests and diagnostic evaluation, and availability of tools and equipment.
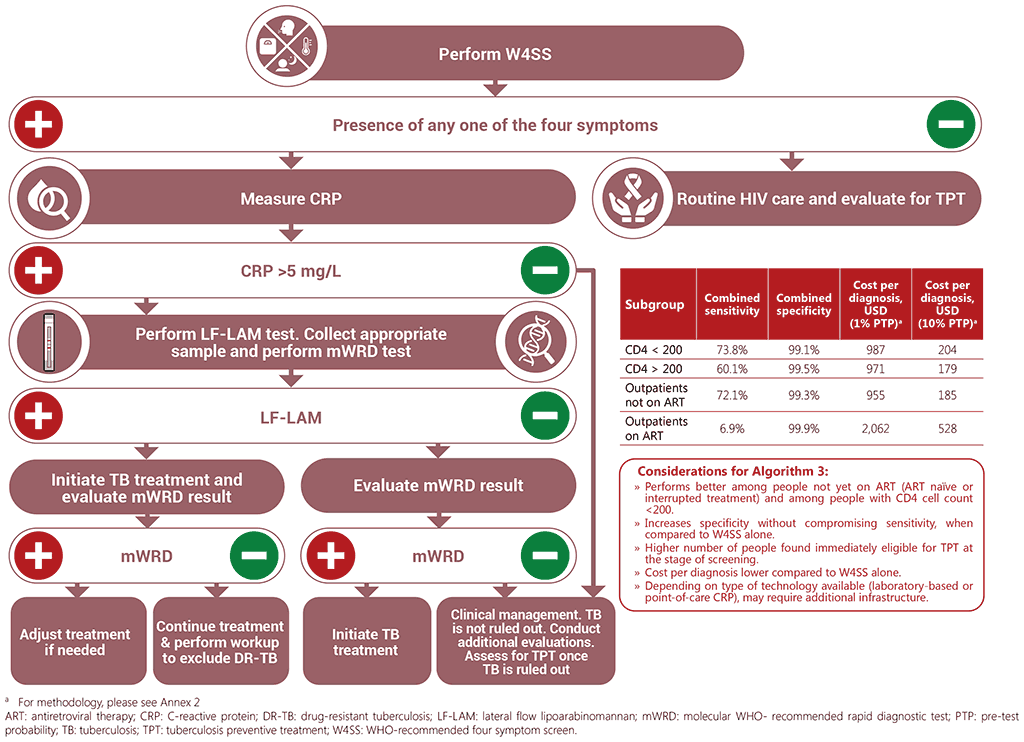
Each of the five screening and diagnostic algorithms presented below is accompanied by data on their sensitivity, specificity, diagnostic yield, and cost per TB diagnosis in given sub-populations. This is to help guide programmes in algorithm selection, according to the strengths and weaknesses of the different algorithms in different populations. Table 3.2 provides a summary of which algorithms may be best used for which population. Selection of algorithms should be informed by clinical presentation, availability of the screening and diagnostic tests, and connection to the required sample transportation networks.
Table 3.2. Summary of algorithms for screening and diagnosing TB among people living with HIV
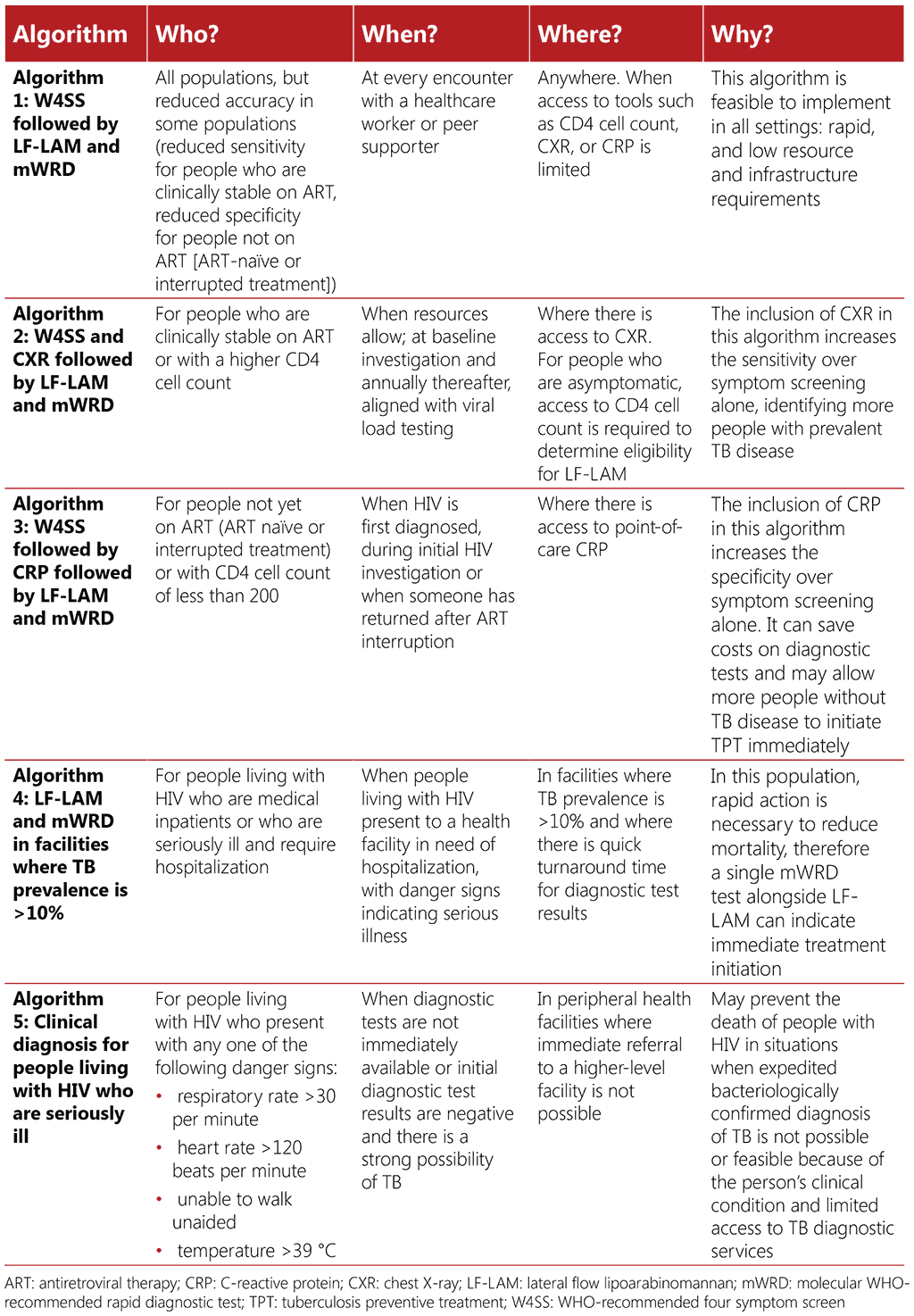
It is important to note the assumptions made when developing these model estimates, which include the fact that all people undergoing screening can produce sputum, that tools are available and used, that there is no loss to follow-up between the screening and diagnostic pathway, and that timely results are received for each test that is undertaken. Costs to implement an algorithm will vary widely between settings, depending on factors such as TB prevalence, availability and cost of tools and equipment, existing infrastructure, personnel costs and healthcare worker training needs. The estimated costs for each algorithm are global cost estimates based on a prevalence of 1% and 10% and may be a guide to comparing relevant costs for the different algorithms, while considering that the actual costs will vary between settings. Details on methods for development of the algorithms, yields, and calculation of algorithm performance and costs, including assumptions, can be found in Annex 2. For country-specific costs, please refer to the ScreenTB web-based tool (https://screentb.org/).
Algorithms for screening and diagnosis of TB among people living with HIV
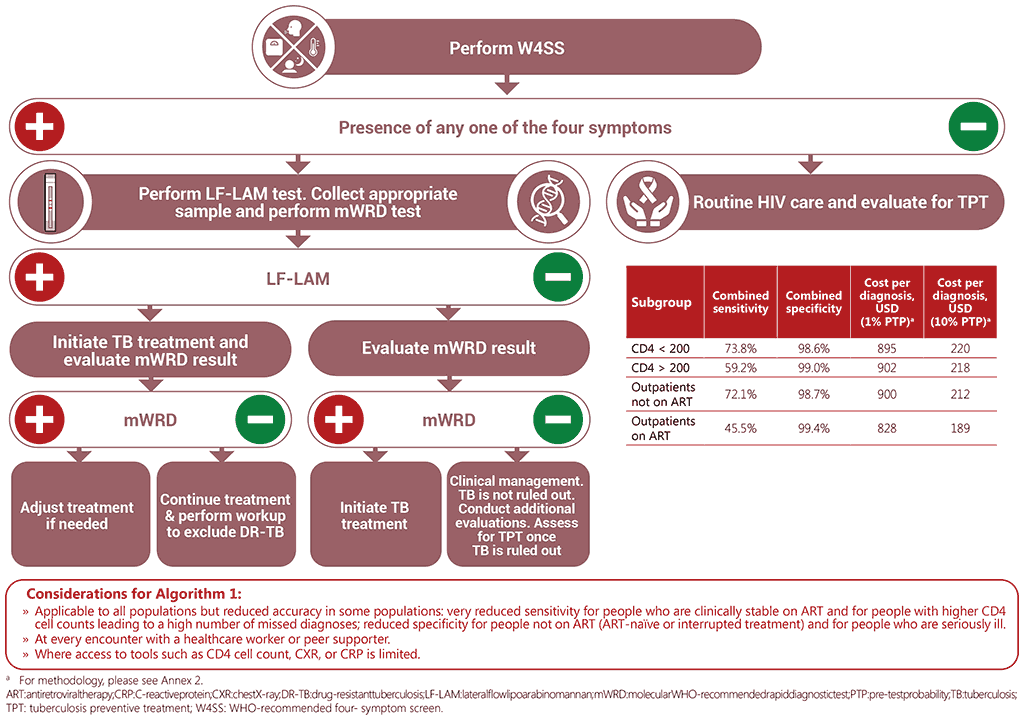
Algorithm 1: W4SS followed by LF-LAM and mWRD
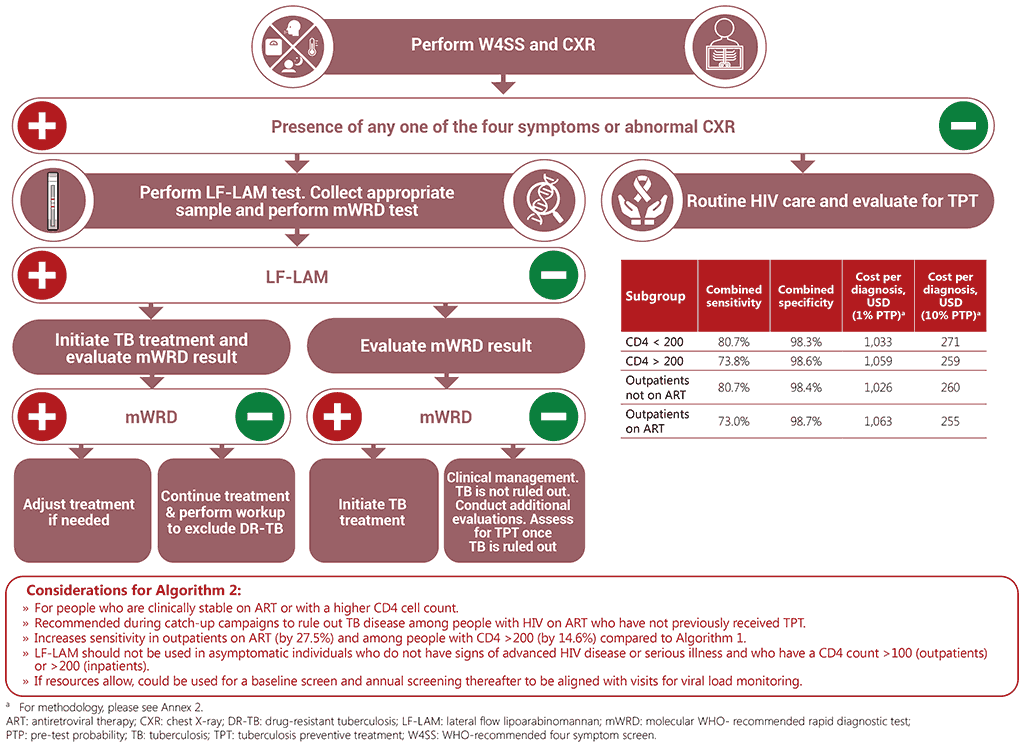
Algorithm 2: W4SS and CXR followed by LF-LAM and mWRD
Algorithm 3: W4SS followed by CRP followed by LF-LAM and mWRD
Algorithm 4: LF-LAM and mWRD for medical inpatients living with HIV or people living with HIV who are seriously ill and require hospitalization in facilities where TB prevalence is >10%
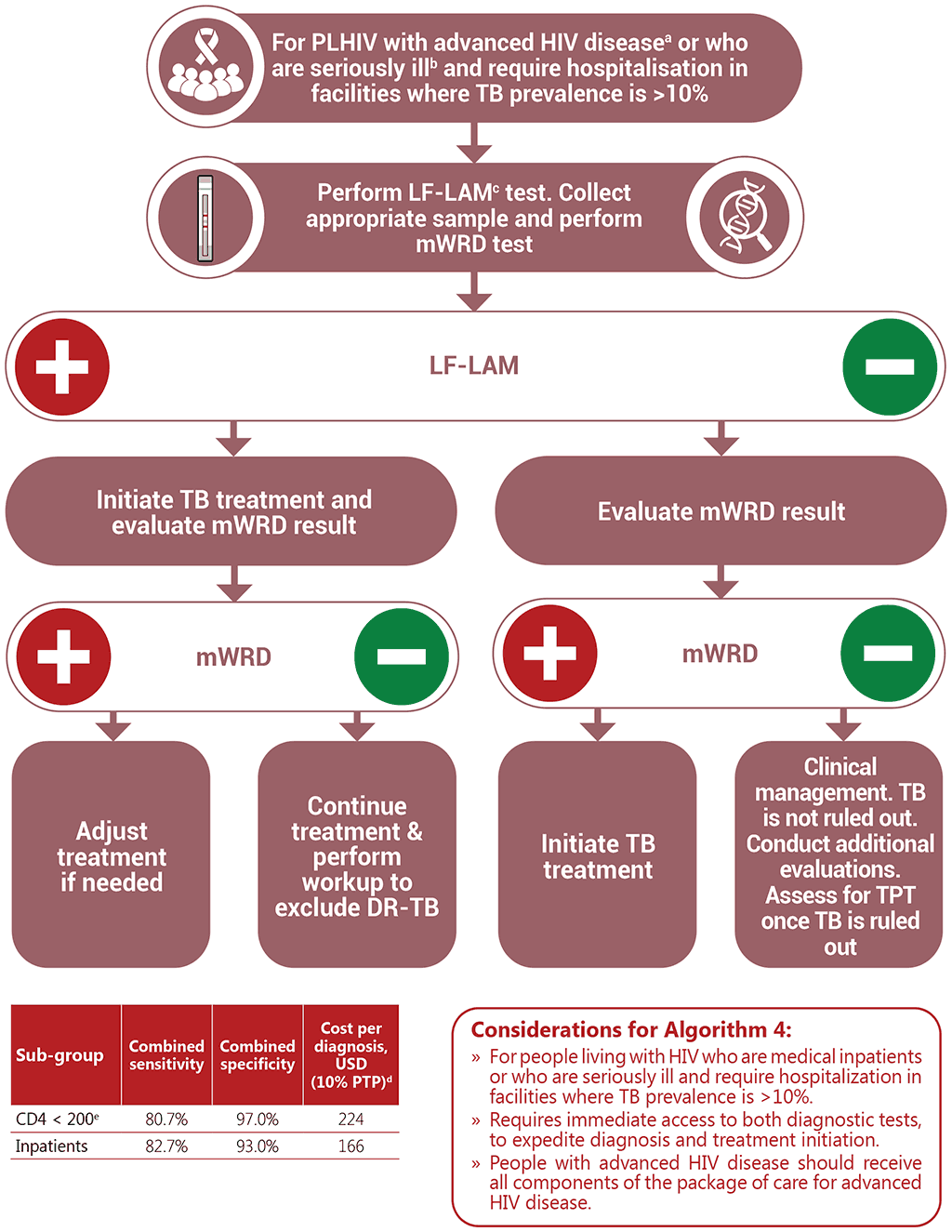
a Advanced HIV disease is defined based on a CD4 cell count <200 cells/mm3 or WHO stage 3 or 4 in adults and adolescents
b Seriously ill is defined based on four danger signs: respiratory rate of more than 30/minute, temperature of more than 39 °C, heart rate of more than 120/minute and unable to walk unaided
c Asymptomatic outpatients with a CD4 cell count <100 are eligible for LF-LAM
d For methodology, please see Annex 2.
e In the absence of data on sensitivity and specificity for people with advanced HIV disease who are seriously ill, CD4 cell count of <200 has been used as a proxy for this subgroup.
DR-TB: drug-resistant tuberculosis; LF-LAM: lateral flow lipoarabinomannan; mWRD: molecular WHO- recommended rapid diagnostic test; PLHIV: people living with HIV; PTP: pre-test probability; TB: tuberculosis; TPT: tuberculosis preventive treatment.
Algorithm 5: Clinical diagnosis of TB in people living with HIV who are seriously ill
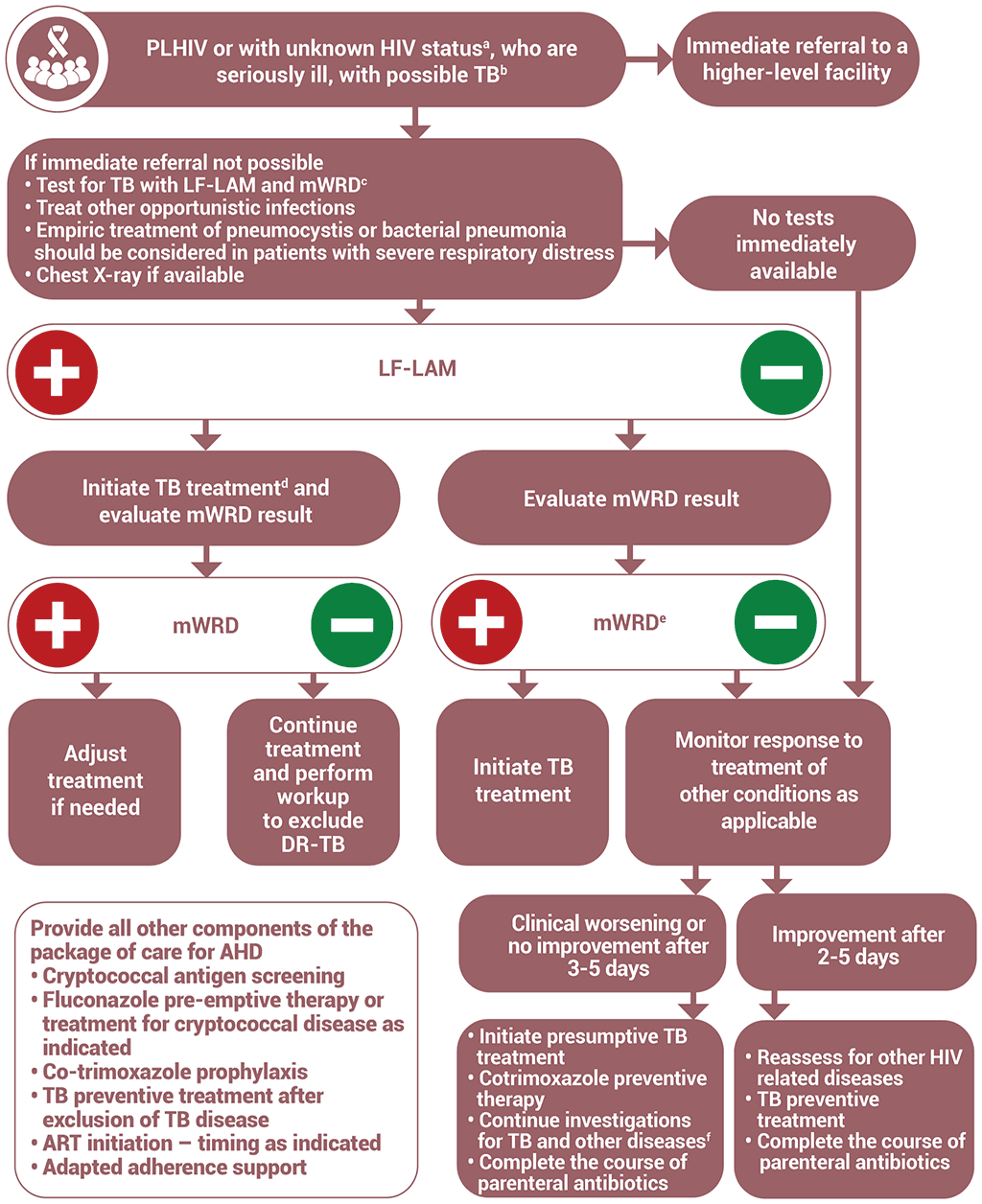
a For all people with unknown HIV status, HIV testing should be performed according to national guidelines.
b Possible TB is defined by the presence of any one of the following symptoms: current cough, fever, weight loss or night sweats. Danger signs are defined as any one of the following: respiratory rate > 30 per minute, heart rate >120 beats per minute, unable to walk unaided, temperature >39 °.
c For people with possible extrapulmonary TB, extrapulmonary specimens should be obtained for mWRD (cerebrospinal fluid, lymph nodes and other tissues: mWRD has low sensitivity for pleural fluid and data are limited for stool, urine or blood). If mWRD is not available, conduct other available TB diagnostic tests and refer appopriate specimens for testing with mWRD and TB culture where feasible.
d If mWRD shows rifampicin resistance, treatment for multidrug-resistant TB should be initiated. If the person is considered at low risk for rifampicin resistance, a second mWRD test should be performed on a fresh specimen. Collect and refer a sample for culture and additional drug sensitivity testing.
e If mWRD shows negative results, the test can be repeated using a fresh specimen.
f Further investigations for TB include chest X-ray, clinical assessment, a repeat mWRD using a fresh specimen and culture. If extrapulmonary TB is suspected, extrapulmonary specimens should be obtained and sent for culture and abdominal ultrasound may be performed.
AHD: advanced HIV disease; ART: antiretroviral therapy; DR-TB: drug-resistant tuberculosis; LF-LAM: lateral flow lipoarabinomannan; mWRD: molecular WHO- recommended rapid diagnostic test; PLHIV: people living with HIV; TB: tuberculosis.
 تعليق
تعليق