Book traversal links for 2.1 Recommendation
Remarks
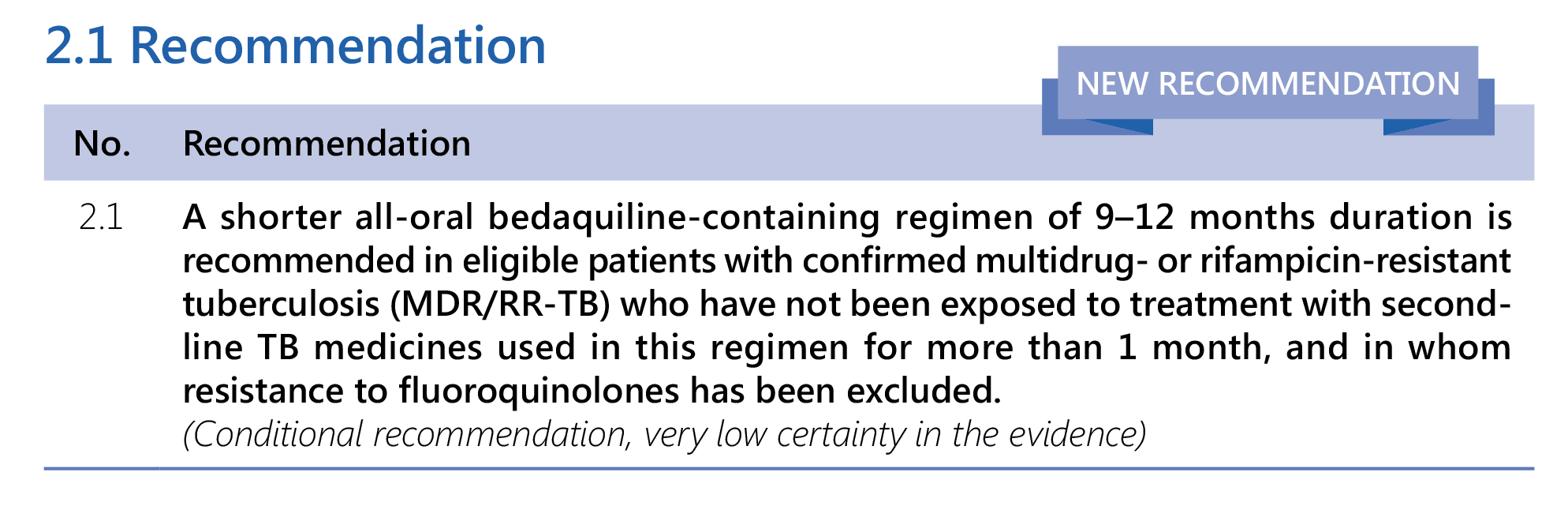
- The 9-month all-oral regimen consists of bedaquiline (used for 6 months), in combination with levofloxacin/moxifloxacin, ethionamide, ethambutol, isoniazid (high-dose), pyrazinamide and clofazimine (for 4 months, with the possibility of extending to 6 months if the patient remains sputum smear positive at the end of 4 months), followed by treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide (for 5 months). Ethionamide can be replaced by 2 months of linezolid (600 mg daily).
- A 9-month regimen with linezolid instead of ethionamide may be used in pregnant women, unlike the regimen with ethionamide.
- This recommendation applies to:
- people with MDR/RR-TB and without resistance to fluoroquinolones;
- patients without extensive TB disease22 and without severe extrapulmonary TB;23
- patients with less than 1 month exposure to bedaquiline, fluoroquinolones, ethionamide, linezolid and clofazimine; when exposure is greater than 1 month, these patients may still receive this regimen if resistance to the specific medicines with such exposure has been ruled out;
- all people regardless of HIV status;
- children (and patients in other age groups) who do not have bacteriological confirmation of TB or resistance patterns but who do have a high likelihood of MDR/RR-TB (based on clinical signs and symptoms of TB, in combination with a history of contact with a patient with confirmed MDR/RR-TB).
Rationale
The rationale for this recommendation is based on the evidence and considerations detailed in the next two subsections. The 9-month regimens can be used in patients not eligible for the shorter, 6-month regimens; also, they represent a preferred treatment option over the longer regimens. The intention to determine a relatively shorter duration of treatment for patients with forms of DR-TB or other eligibility criteria not compatible with the 6-month regimen has driven the assessments presented in this section.
Briefly, two assessments have been performed: first, comparing the outcomes of the 9-month regimen including linezolid for 2 months and the identical regimen that included ethionamide for 4 months; and second, comparing the outcomes of the 9-month regimen including linezolid with the longer regimens that were designed individually but included both bedaquiline and linezolid along with other medicines as recommended by WHO. Data on most of the 9-month regimens were obtained from a programmatic setting in South Africa.
The first assessment showed similar levels of treatment success (64% vs 66%), failure or recurrence (1.1% vs 1.4%), deaths (20% vs 21%), loss to follow-up (15% vs 12%) and amplification of drug resistance (0.6% vs 0%). Adverse events were noted in 5% of participants receiving the 9-month regimen with linezolid; however, no comparisons could be made because no data were available for participants receiving the 9-month regimen with ethionamide. The second assessment of the 9-month regimen compared with longer regimens also showed lower levels of treatment success (64% vs 74%), failure or recurrence (1% vs 3%) or amplification of drug resistance (1% vs 2%); and higher levels of deaths (20% vs 11%) or loss to follow-up (15% vs 12%). Adverse events were noted in 5% of participants receiving the 9-month regimen with linezolid and in participants receiving longer regimens.
Based on a combined review of these two assessments it was considered that the 9-month regimen with linezolid can be recommended as an alternative to the 9-month regimen with ethionamide, and that both regimens can be used in preference to the longer (18-month) regimens in eligible patients. These assessments were performed on the background of the previous assessment during the GDG meeting in 2019 that led to the conditional recommendation for use of the 9-month all-oral bedaquiline-containing regimen (41). The datasets of both 9-month regimens systematically excluded patients with extensive TB disease and severe forms of extrapulmonary TB; therefore, this recommendation is not extended to these groups of patients.
22 Extensive (or advanced) pulmonary TB disease is defined as the presence of bilateral cavitary disease or extensive parenchymal damage on chest radiography. In children aged below 15 years, advanced disease is usually defined by the presence of cavities or bilateral disease on chest radiography.
23 Severe extrapulmonary TB is defined as the presence of miliary TB or TB meningitis. In children aged below 15 years, extrapulmonary forms of disease other than lymphadenopathy (peripheral nodes or isolated mediastinal mass without compression) are considered to be severe.
 تعليق
تعليق