Book traversal links for 3.1 TB screening
3.1.1 Frequency of screening
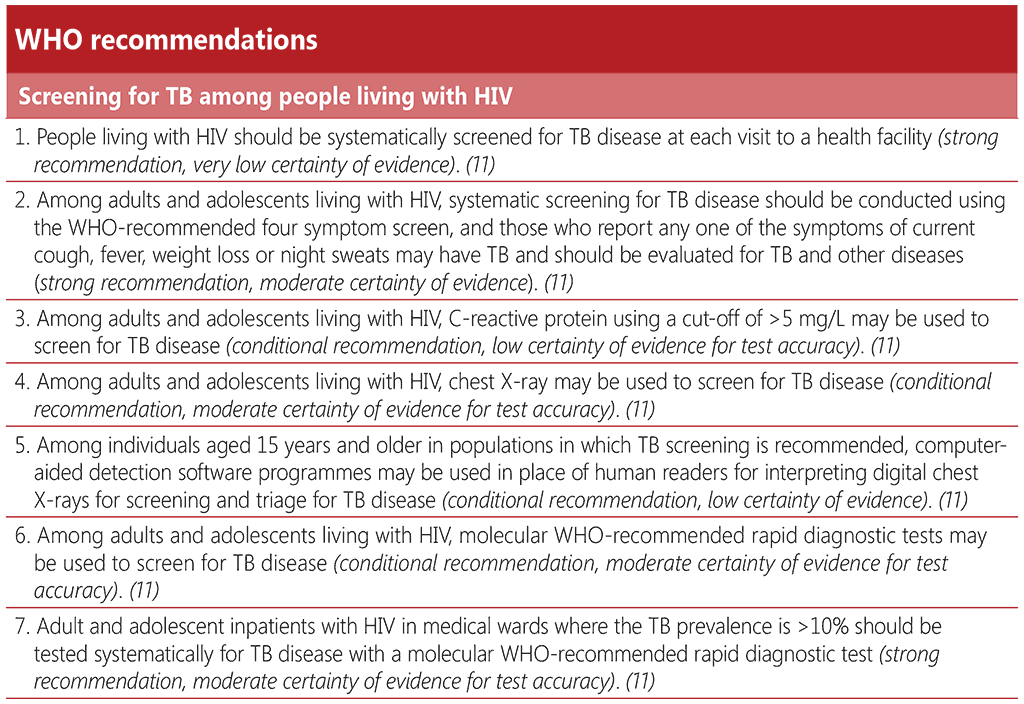
WHO recommends that people living with HIV should be screened for TB disease at each visit to a health facility (73). In addition to the WHO-recommended four symptom screen (W4SS), three more TB screening tools are now recommended for people living with HIV, namely, chest X-ray (CXR), C-reactive protein and molecular WHO-recommended diagnostic tests. These may be used alone or in combination with the W4SS, depending on the resources available. At a minimum, a W4SS should be conducted and this may be supplemented with additional screening tools, for instance at the time of initial HIV diagnosis or during the first antenatal care visit for pregnant women, and then annually thereafter.
3.1.2 Screening tools
All the screening tests described here identify adults and adolescents with HIV with a higher probability of TB disease and who should be referred for diagnostic evaluation. When TB disease is ruled out, the individual should be referred for evaluation for TB preventive treatment.
WHO-recommended four symptom screen
The W4SS comprises the following symptoms: current cough, fever, night sweats or weight loss. It was first recommended in 2011, with an initial recommendation for systematic screening of all people living with HIV at every visit to a healthcare facility, primarily for ruling out TB disease, due to its high negative predictive value. However, if adults and adolescents with HIV screen positive for any one of these symptoms, further diagnostic workup is recommended. W4SS is a simple screening approach that is non-invasive and is feasible to implement repeatedly in any setting, by any level of healthcare provider. The results of a symptom screen may be subjective and depend on the end user’s level of understanding and willingness to share their physical experience of symptoms, as well as on the provider’s interpretation of the reported symptoms. Thus, the quality and consistency of the W4SS is likely to vary between clinical settings.
An individual participant data meta-analysis that informed the 2021 WHO screening guideline revision found that the sensitivity of the W4SS among all people living with HIV was 83% (95% CI: 74–89%) and specificity was 38% (95% CI: 25–53%). When used alone, the sensitivity of the W4SS was lowest among outpatients on ART and among pregnant women, and it had markedly low specificity among medical inpatients and individuals not receiving ART (ART naïve or interrupted treatment) (11). The W4SS has an important role in ruling out TB disease due to its high negative predictive value in most settings. This is important in the preventive TB care pathway of people living with HIV who would benefit from receiving TPT in the absence of TB disease.
While there may be real-life limitations to the W4SS in terms of consistency and quality of delivery that might not be reflected in studies, the W4SS is an essential part of the clinical examination of most subpopulations and is the most accessible screening tool at all levels of the health system. It can be repeated as often as necessary, while more intense screening strategies with additional screening technologies might be used less frequently, such as at initial HIV diagnosis and at annual check-ups such as for viral load monitoring. Familiarity with W4SS is already widespread in many HIV services, as a result of capacity-building efforts and supervision.
It is however important to continuously monitor and enhance the quality of delivery of the W4SS in all settings, including within differentiated service delivery. This can be done through training of healthcare workers and peer workers, and through operational research. Studies to monitor quality may include tracking screening positivity rates, the use of auditing tools and exit interviews among people attending HIV care as well as studies to monitor the cascade of screening, diagnosis and care (74, 75). Indicators listed in Annex 1 can assist programmes in assessing the gaps in the cascade, from screening through to diagnosis and initiation of TB treatment and TB preventive treatment.
C-reactive protein
C-reactive protein (CRP) is an indicator of systemic inflammation that can be measured with a blood test. CRP offers an improvement in accuracy over the W4SS among outpatients with HIV who are not yet on ART (CRP, sensitivity 89% and specificity 54%; W4SS, sensitivity 84% and specificity 37%). CRP at cut-off values of either >5 mg/L or >10 mg/L is similar to or more accurate than W4SS, depending on the subpopulation tested (76). The cut-off of >5 mg/L is recommended because it is the lowest threshold that indicates abnormality in many clinical settings and is more sensitive. At this cut-off, CRP has a similar sensitivity and higher or similar specificity to symptom screening in all subpopulations of adults and adolescents with HIV. The choice of cut-off value will, however, depend on the type of CRP technology available, the prevalence of other conditions that may increase CRP values and a preference for increased sensitivity or increased specificity.
CRP can be used on its own or in combination with the W4SS. The parallel use of two screening tools, whereby a positive screen for either tool leads to a diagnostic test, will have resource implications because of the higher sensitivity and lower specificity. However, data reviewed for the 2021 guideline revision supports the sequential combination of a positive W4SS followed by CRP (with a prespecified cut-off of >5 mg/L), particularly for people living with HIV not yet on ART, for whom it has a sensitivity of 84% (95% CI: 73–90%) and a significantly higher specificity of 64% (95% CI: 55–72%). CRP can play an important role in ruling out TB disease before initiation of TPT. Screening with the W4SS followed by CRP increases the number of true negatives compared with the W4SS alone, thus increasing the number of people eligible for TPT. An additional potential benefit of CRP is that it can alert clinicians to the presence of other infectious or non-infectious conditions. Health staff and individuals being screened may be more confident in the results of a biochemical test than of a more subjective symptom screen (76).
Currently, many analysers are available for measuring CRP, with different levels of detection, although all can be used for TB screening with a CRP cut-off between 5 and 10 mg/L. The results obtained with most quantitative point-of-care analysers to measure CRP, correlate strongly with those of laboratory analysers. Point-of-care CRP tests provide rapid (≤5 min) quantitative results from a capillary blood sample. Hence, they do not require phlebotomy, are simple enough to be performed by frontline healthcare workers after minimal training and do not require connection to a laboratory transportation network for analysis. Some semi-quantitative test strips are available with operational characteristics, ideal for use in remote settings (inexpensive, no analyser required); however, the agreement of results with those of laboratory analysers is moderate and may decrease further if time-to-test strip interpretation exceeds 5 min.
Containers for safe disposal of needles and lancets must be available, and other infection control measures in the collection of blood must be followed. The overall laboratory requirements are minimal; however, most analysers require a continuous electricity source, and most CRP assays require cold storage and refrigeration (+2 to +8 °C). If point-of-care testing for CRP is not available, blood samples will have to be sent to the nearest laboratory, which will significantly undermine the utility of the test for on-the-spot decision-making and render it less useful for screening in outpatient settings.
Chest radiography
Chest X-ray is currently recommended by WHO for use in parallel with the W4SS for ruling out TB disease before initiating TPT. Similarly, CXR can be used in parallel with the W4SS to screen for TB disease, a positive or abnormal result on either screen indicating referral for diagnostic evaluation. Reading modalities of “any abnormality” or “abnormality suggestive of TB” can be used, depending on the context, the availability of radiological expertise, resources and a preference for higher sensitivity or higher specificity.
CXR alone was found to have similar sensitivity to and similar or higher specificity than the W4SS across all subpopulations of people living with HIV. A combined parallel screening strategy of the W4SS and CXR offers a significant improvement in sensitivity (93% for W4SS only vs 53% for W4SS and CXR in parallel), particularly for screening outpatients enrolled in ART care, over the W4SS alone, although with lower specificity. However, in some subgroups like inpatients and people with advanced HIV disease, the specificity is very low.
CXR requires interpretation by a radiologist, other trained health personnel or computer-aided detection (CAD) software. CAD products, which were first recommended by WHO in 2021 (11), use artificial intelligence to analyse CXR images for the presence of abnormalities suggestive of pulmonary TB, producing an abnormality score that can be used to determine the need for followon diagnostic testing for TB relative to a selected threshold. Access to and scale-up of CAD has been facilitated by the advent of digital radiography. CAD technology can improve the feasibility and performance of CXR for screening and triage for TB disease by enhancing capacity for TB screening. Such technology can replace or augment human expert interpretation of plain CXR when screening for TB and can avoid inter-reader variability and reduce delays in reading radiographs when skilled personnel are scarce (77).
Further information on CAD for the interpretation of CXR can be found in the consolidated guidelines and operational handbook on TB screening (11, 76). CXR findings may differ widely in people living with HIV-associated TB, from a completely normal picture to multiple radiological abnormalities typically associated with advanced TB disease (78).
Although no data are available on the optimal periodicity of CXR screening, a pragmatic approach would be to perform CXR annually among outpatients with HIV at the time of viral load testing or other investigations, in addition to W4SS at every encounter with a health worker between annual screens. A baseline CXR and access to imaging taken previously are useful for comparing subsequent radiological changes.
Although CXR is the preferred screening tool when combined in parallel with the W4SS, from the viewpoint of test sensitivity, it is important to consider how costs can be mitigated. Programmes should consider eliminating out-of-pocket costs for CXR or using vouchers to further reduce barriers to accessing this critical tool for TB control. Where HIV services are not co-located with TB and radiography services, programmes should consider providing funding for people to travel for CXR or using mobile screening to improve access to CXR screening (79).
As with all TB screening, it is essential to engage with and provide information to local civil society organizations and primary care providers to enhance screening uptake and performance. Chest X-ray is most relevant for people living with HIV who are clinically stable on ART, in care, are immunocompetent and likely to be supported in the community. The risks of exposure to ionizing radiation might be a greater concern for this group, particularly if they undergo CXR regularly and may also receive radiography to evaluate health problems between screenings. Direct CXR is a safe technology using a radiation dose of 0.1 mSv, which corresponds to 1/10 of the annual accepted dose of ionizing radiation for the general public (1 mSv) (80). Therefore, exposure to the low radiation doses delivered during a chest X-ray poses a minimal risk of inducing tissue reactions or cancer in the years or decades following the examination (81). Furthermore, CXR does not pose any significant risk for pregnant women or the fetus, provided that good practices are observed, with the primary beam targeted away from the pelvis.
Molecular WHO-recommended rapid TB diagnostic tests
There is now a conditional recommendation for the use of molecular WHO-recommended rapid TB diagnostic tests (mWRDs) in TB screening among people living with HIV. Implementation of an mWRD as a screening tool will require significant resources, including increased capacity and expansion of diagnostic and sample transportation networks. There has been limited experience in widescale use of mWRDs for screening under programmatic conditions. However, depending on feasibility and available resources, countries may choose to adopt a targeted approach to TB screening with mWRDs in certain subpopulations such as people with advanced HIV disease, or pregnant women living with HIV. Priority should be given to ensuring universal access to mWRDs as a diagnostic test for TB and drug-resistant TB before extending its use to screening. The accuracy of mWRDs in most subpopulations is not significantly different from that of a W4SS followed by an mWRD. Screening with an mWRD in lower prevalence settings may result in higher false positives should the diagnosis not be confirmed, with the associated overtreatment and related social and economic consequences, including potential delays in starting ART
People who screen positive for TB with an mWRD should always receive a thorough clinical evaluation, including symptom screening and further tests, such as CXR or repeat mWRDs on additional sputum samples, to establish a definitive diagnosis of TB (76). Among medical inpatients in settings where the prevalence of TB is ≥10%, mWRDs are strongly recommended as part of rapid diagnostic workup, because of the severity of illness in this population. As rapid diagnosis and care are required in this particular subpopulation, a positive mWRD result can be considered an indication for treatment and need not be followed by a separate diagnostic evaluation. It is also essential to ensure proper monitoring of treatment response and evaluation for alternative diagnoses.
Several considerations apply to the use of mWRDs as a screening tool. mWRDs perform differently when used for screening than when used for diagnosis. Because of the differences in accuracy and the lower TB prevalence typically found in a population undergoing screening rather than diagnostic evaluation, the positive and negative predictive values of mWRDs also differ. For example, despite a high estimated specificity of 99%, over one half of positive screening tests will be false-positive when mWRDs are used to screen a population with a 1% prevalence of TB. Thus, the different implications for clinical interpretation and programmatic use of mWRDs for screening and for diagnosis must be understood. In addition, clinicians may wish to exercise their clinical judgement when interpreting mWRD results in light of the findings of a clinical examination, patient history, as well as results from other tests.
For people who have had TB in the previous 5 years, a positive mWRD result may be due to the detection of DNA persisting from the earlier TB episode. Therefore, a positive test in such cases should be investigated with phenotypic methods to exclude a false-positive result (12). A negative mWRD for a single sputum sample does not exclude TB, as individuals with TB may test mWRDnegative because they cannot produce an adequate quantity of sputum or any at all, have a very low bacillary burden in the sample or have extrapulmonary disease. If a person is unable to provide sputum, other TB screening strategies should be considered.
If use of mWRDs for screening requires decentralization of the technology, there may be significant implications in terms of the purchase of machines, cartridges and other consumables, the need for an uninterrupted supply of electric power, and maintenance. If mWRD technology does not reach most health centres, samples will have to be transferred; in this instance, shifting from mWRDs for diagnosis to screening would substantially increase the workload for sample transport systems. Diagnostic connectivity platforms that automate the transmission, storage and retrieval of test results will improve the utility of mWRDs for decision-making.
 تعليق
تعليق