Links de passagem do livro para Annex 2. Methods and expert panels
WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment, second edition
A2.1 Scope and objectives
The WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment make recommendations for the four milestones of the cascade of preventive care, namely, identification of risk groups, TB screening and ruling out TB, testing for TBI and choice and administration of the TPT regimen. The second edition of the TPT guidelines covers the same milestones.
Since the previous update of the guidelines on TPT, in 2020 (1), further developments have occurred that affect TPT policy. They include revision of WHO guidance on screening for TB disease and new modalities for testing for TBI (2,3). In addition, by 2023, two landmark trials of TPT for contacts of patients with MDR-TB had been completed (4,5). In view of this new information and continued demand by Member States for guidance on protecting people at risk of TB, a second edition of the TPT guidelines has been prepared that includes the latest evidence. The objectives of this second edition were to:
- review the latest evidence for TPT in cases of MDR-TB and revise the respective recommendation accordingly;
- align the guideline recommendations on ruling out TB and testing for TBI to the WHO recommendations on screening and diagnostics that have been revised since 2020; and
- enhance the operational guidance with more practical details on dosing schedules, support for adherence to medication and minimizing the toxicity of current regimens.
The aim of the revised guidelines is to support more effective global scaling up of TPT and to contribute to ending the global TB epidemic. These updated guidelines will allow users to choose the management approach best suited for all target groups in each context. It also provides a sound basis for the development or updating of national guidelines for TPT, which is based on the epidemiology of TB and the health-care delivery system in each country. Furthermore, the guidelines address the request by Member States for a comprehensive policy and operational guidance for programmatic management of TPT. The guidelines are being issued with an updated operational handbook containing complementary, practical details for implementation.
A2.2 Methods used to develop the guidelines
In accordance with the process recommended by the Guideline Review Committee (6), three expert groups were established: a Guideline Steering Group, composed of WHO staff; the GDG, comprising external content experts, national TB programme managers, other implementers, academics, researchers and representatives of patients and civil society, led by a guideline methodologist; and the ERG, composed of peer-reviewers.
The WHO Guideline Steering Group prepared the background document for the guidelines, which detailed the PICO question that would define the main evidence-based recommendation that was to be updated; the trial data and evidence review required; draft changes to the wording of existing recommendations and accompanying remarks to improve clarity and implementation of the guidance; and the composition of the expert panels. The scoping document was submitted to the Guideline Review Committee and approved in May 2023. Information about the GDG members was placed on a public website in November 2023 (https://cdn.who.int/media/docs/default-source/hq-tuberculosis/biographies_gdg_tpt_2023.pdf?sfvrsn=95176f7e_3).
GDG meetings were conducted as 3-h virtual webinars on 4–6 December 2023, and three virtual preparatory meetings of the GDG were held in September, October and November 2023 to discuss the procedures to be followed and to review the preliminary data. Evidence summary tables were drafted for the PICO question by the guideline methodologist with the GRADE approach and circulated to the group before the webinars. The meetings were chaired by a technical expert, while the guideline methodologist facilitated the discussions to reach consensus, which was defined as unanimous or majority agreement. The GDG agreed in advance that, if unanimity was not achieved for a recommendation to be made, the members of the GDG would vote and that a majority of 60% or more of voting members would be necessary to accept a recommendation. If the vote reached this threshold but was less than 70%, the recommendation would be conditional. The estimates of effect and the judgements on the quality of evidence were reviewed by the GDG during the online discussions. GRADE evidence-to-decision tables were used to guide discussions of benefits and harm, the quality of evidence, cost, feasibility, acceptability, equity, values and preferences. The direction of the recommendation and its strength (strong or conditional) were determined by these factors. GRADEpro was used to document the decisions made (7).
A2.3 Scoping and PICO question
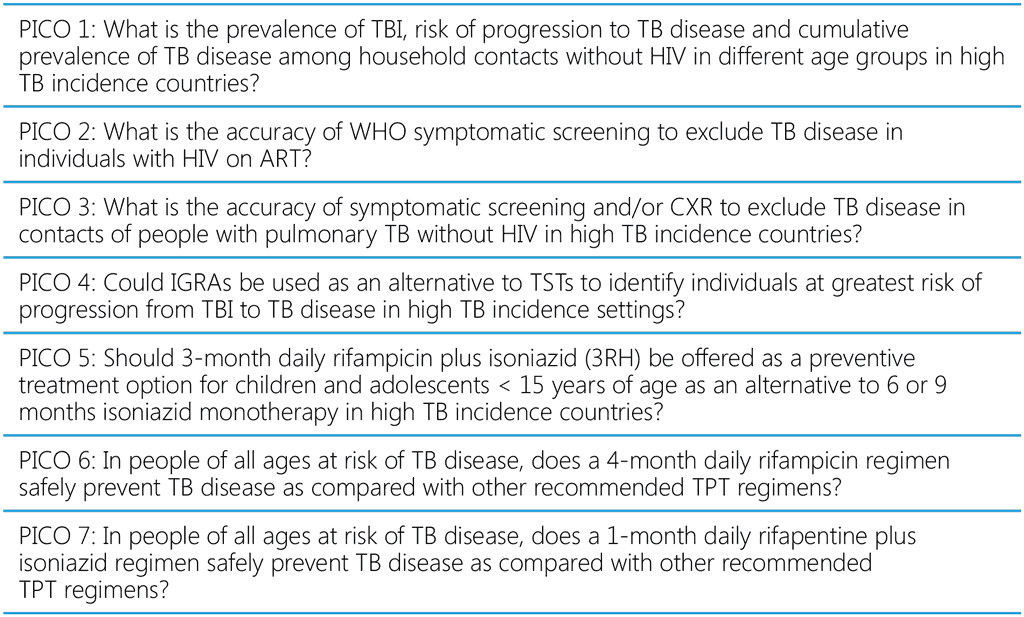
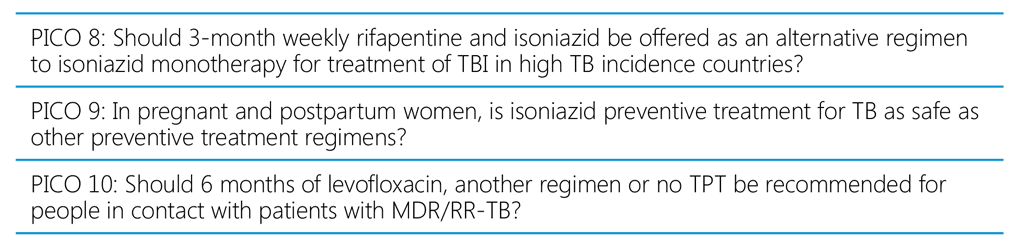
The recommendations in the WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment, second edition are structured around 10 PICO questions (Table A2.1).
Table A2.1. PICO questions for the WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment, second edition
Evidence retrieved for the second edition of the TPT guidelines was primarily to answer PICO question 10 (Table A2.2). The answers to this question were intended to be used to update the original conditional recommendation on TPT of MDR-TB, which was based on very low certainty of the estimates of effect, namely: “In selected high-risk household contacts of patients with multidrugresistant tuberculosis, preventive treatment may be considered based on individualized risk assessment and sound clinical justification”.
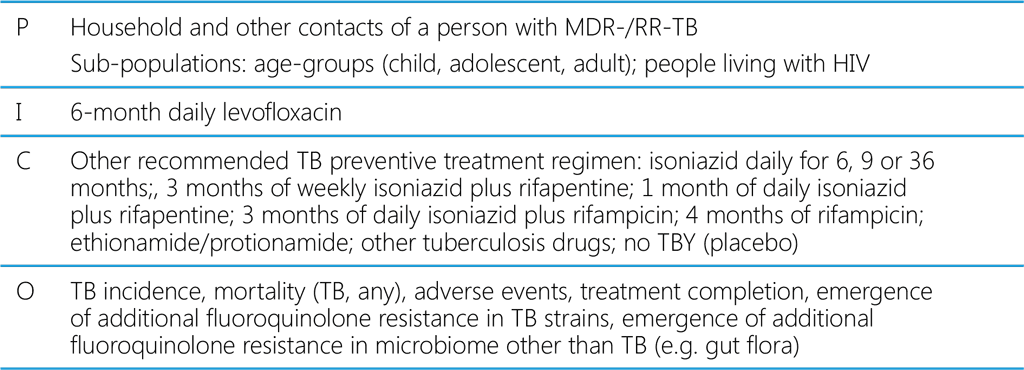
Table A2.2. PICO question on TB preventive treatment for contacts exposed to MDR/RR-TB: Does tuberculosis preventive treatment with levofloxacin improve outcomes in contacts exposed to multidrug- or rifampicin-resistant tuberculosis when compared with other regimens or no treatment?
Once the PICO question had been finalized by the GDG, a list of potential outcomes of interest was circulated to all members to score the importance of each outcome on an incremental scale of 1–9: 1–3: “not important”; 4–6: “important”; and 7–9: “critical”. The mean of the scores for each outcome was then used to prioritize those for evidence summarization and for GDG discussions. All outcomes were scored by the GDG as “critical” or “important” (see also the GRADE tables in annexes 3 and 4).
Most of the evidence reviewed for the main outcomes of this PICO question was from two randomized, placebo-controlled trials on use of levofloxacin vs no treatment (4,5). A literature search was also conducted for other published studies that could inform the recommendation. In addition, a survey of users in national TB programmes and of people in contact with MDR-TB was conducted on various aspects of implementation (e.g. acceptability, feasibility, impact on equity).
In addition to the review of evidence for the PICO question, the previous recommendations were reviewed for clarity of wording, applicability in different settings and alignment with other WHO guidance. The structure used in the first edition of the guidelines, in 2020, which was the cascade of programmatic management of TPT, was retained. This is: identification of populations at risk (adults and children living with HIV, adult and child contacts of people with TB and other risk groups); ruling out TB disease; testing for TBI; providing treatment (including managing adverse events and supporting adherence) and monitoring and evaluation. The text of the recommendation is followed by summaries of the evidence (justification), discussion of their rationale and considerations on implementation, key subgroups, monitoring, evaluation and research gaps. Recommendations that remained valid were retained, with or without slight rewording (see Annex 1, above). Two that were considered obsolete by the GDG were withdrawn. Relevant recommendations from two WHO guidelines in the consolidated series that were issued in 2021 and 2022 were included in this second edition of the guidelines. (For methods used in previous guidelines, see the respective documents and related annexes (1–3,8).)
The guidelines and the supporting documents were reviewed and endorsed by all GDG members. Remarks from the ERG were assessed by the WHO Guideline Steering Group and included in the guidelines. Final approval of the guidelines by the Guideline Review Committee was received on 28 May 2024.
A2.4 Certainty of the estimates of effect and strength of the recommendations
The certainty of the estimates of effect (or the quality of evidence) and the strength of the recommendations were assessed with the GRADE method (9). Certainty of evidence was defined as the degree of confidence that the estimates of effect (desirable or undesirable) are close to the actual effects of interest. The usefulness of an estimate of effect depends on the degree of confidence in that estimate: the higher the certainty of the evidence, the more likely it is that a strong recommendation can be made. WHO guideline development is based on specific criteria for assessing the characteristics of a study, such as within-study bias (methodological quality), consistency, precision, directness or publication bias. Most of the evidence reviewed by the GDG in December 2023 was from two RCTs, which was considered to be of high certainty for five of the eight outcomes and moderate, low or very low for one outcome each (see annexes 3 and 4). An assessment of the risk of bias was conducted by the guidelines methodologist.
The strength of a recommendation reflects the degree of confidence of the GDG that the desirable effects outweigh the undesirable effects. The desirable effects include beneficial health outcomes (e.g. prevention and early diagnosis of TB, reduced TB-related morbidity and mortality), a smaller burden of TB and greater savings. The undesirable effects included harm, a greater burden and greater cost. The “burdens” included adherence to recommendations by programmes, patients and caregivers – formal or informal – such as more frequent tests and taking additional medications.
The certainty of the estimates of effect (quality of evidence) was categorized into four levels:
High: The GDG is very confident that the true effect lies close to that of the estimate of the effect.
Moderate: The GDG is moderately confident that the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: The confidence of the GDG in the effect estimate is limited: the true effect may be substantially different.
Very low: The GDG has very little confidence in the effect estimate: the true effect is likely to be substantially different.
The recommendations are either strong or conditional.
A strong recommendation is one for which the GDG was confident that the desirable effects of adherence to it would outweigh the undesirable effects. The recommendation could be either in favour of or against an intervention.
A conditional recommendation is one for which the GDG concluded that the desirable effects of adherence to it would probably outweigh the undesirable effects; however, the GDG was not confident about the trade-off. The reasons for lack of confidence included: absence of high-quality evidence (few data to support the recommendation); imprecise estimates of benefit or harm (new evidence might change the ratio of risk to benefit); uncertainty or variation in the value of the outcomes for different individuals (applicable only to a specific group, population or setting); and small benefits or benefits that might not be worth the cost (including the cost of implementing the recommendation).
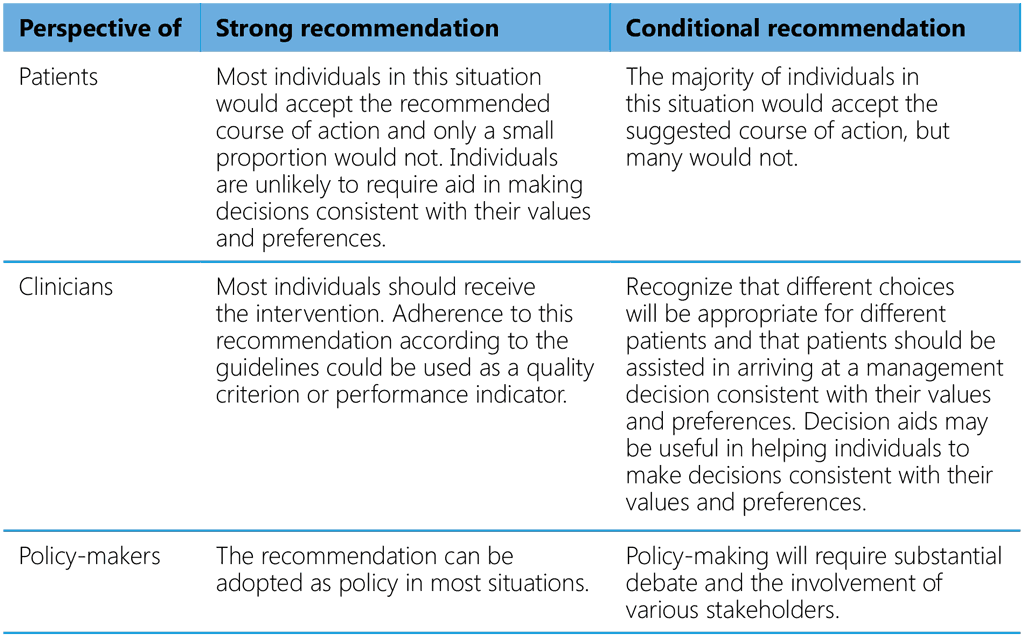
The strength of a recommendation is determined by the balance between desirable and undesirable effects, values and preferences, resource use, equity considerations, acceptability and the feasibility of implementing the intervention. The strength of a recommendation has specific implications for individuals affected by these guidelines (Table A2.3).
Table A2.3. Implications of the strength of a recommendation for different stakeholders
A2.5 Publication, implementation, evaluation and expiry
These guidelines were prepared in accordance with the requirements of the Guideline Review Committee. They are being published for free download on the WHO institutional repository for information sharing (10) and the WHO TB Knowledge Sharing Platform (11) as part of the modular series of WHO consolidated guidelines on TB. The documents will also be communicated widely at international and regional conferences and meetings of programme managers in all regions. They are accompanied by an operational guide containing practical details to support programmatic implementation of the revised recommendations (12).
National programmes will be supported by WHO and technical and funding partners in preparing national plans for programmatic management of TPT, including prioritization of groups at high risk according to local epidemiology and the characteristics of the health system. Implementers should create a conducive policy and programmatic environment, including national and local policies and standard operating procedures to facilitate implementation of the recommendations in these guidelines. This should include promoting universal health coverage and offering public financing for TPT. Furthermore, dedicated resources should be allocated, including for staff development and service delivery in the community. Training of front-line health-care staff and students in critical areas, such as identification of populations at risk, administering tests for TBI, choosing TPT, counselling and management of adverse drug reactions, is important. National programmes should ensure meaningful engagement with affected populations, their communities, the private sector, other relevant health programmes and ministries in both planning and implementing the interventions. The process should facilitate concordance with other guidance on relevant risk factors for TB, such as diabetes, undernutrition and tobacco smoking, and access to comprehensive care for people with these co-existing risks.
The uptake of these WHO recommendations will be monitored during annual data collection for WHO Global TB Data Monitoring (13). WHO will update the guidelines 5 years after their publication or earlier if new evidence becomes available that necessitates a revision.
A2.6 Composition of the Guideline Development Group and the External Review Group6
The following experts composed the GDG and ERG for the second edition of the TPT guidelines (Tables A2.4 and A2.5).
Table A2.4. Guideline Development Group, 2023–2024
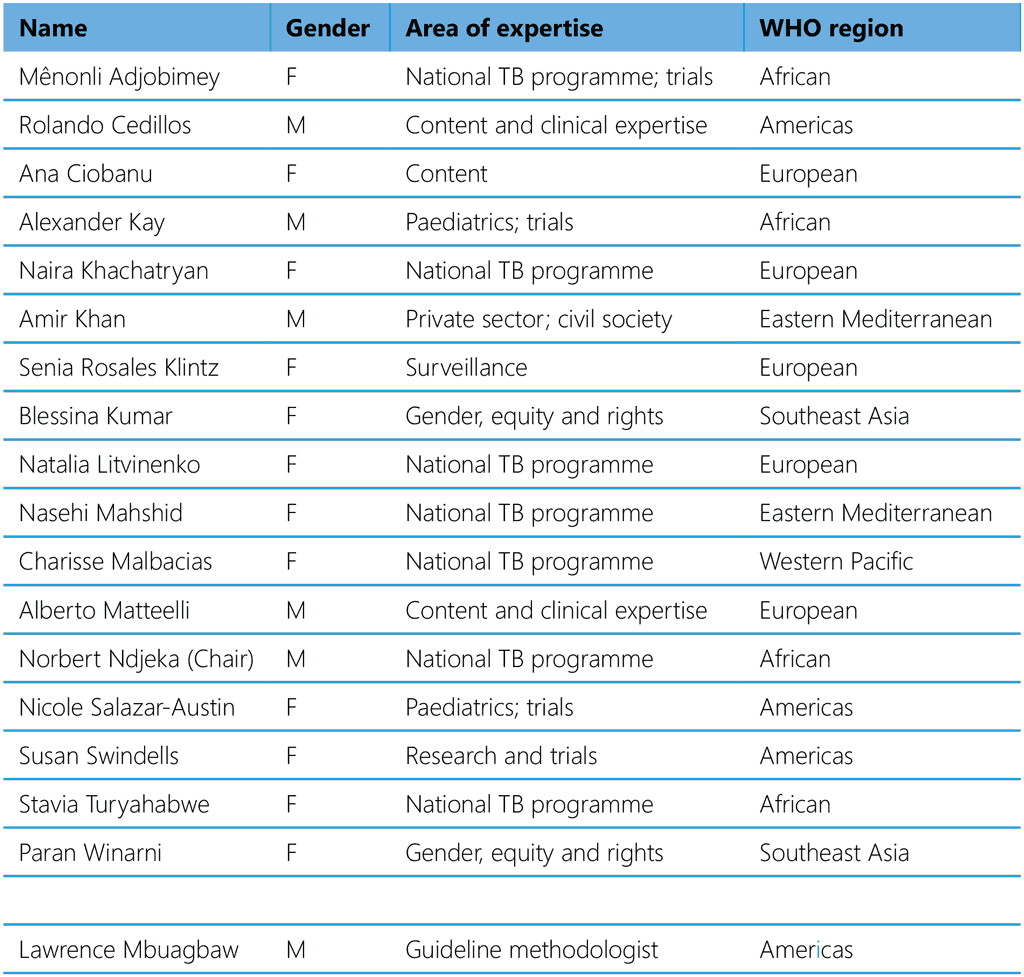
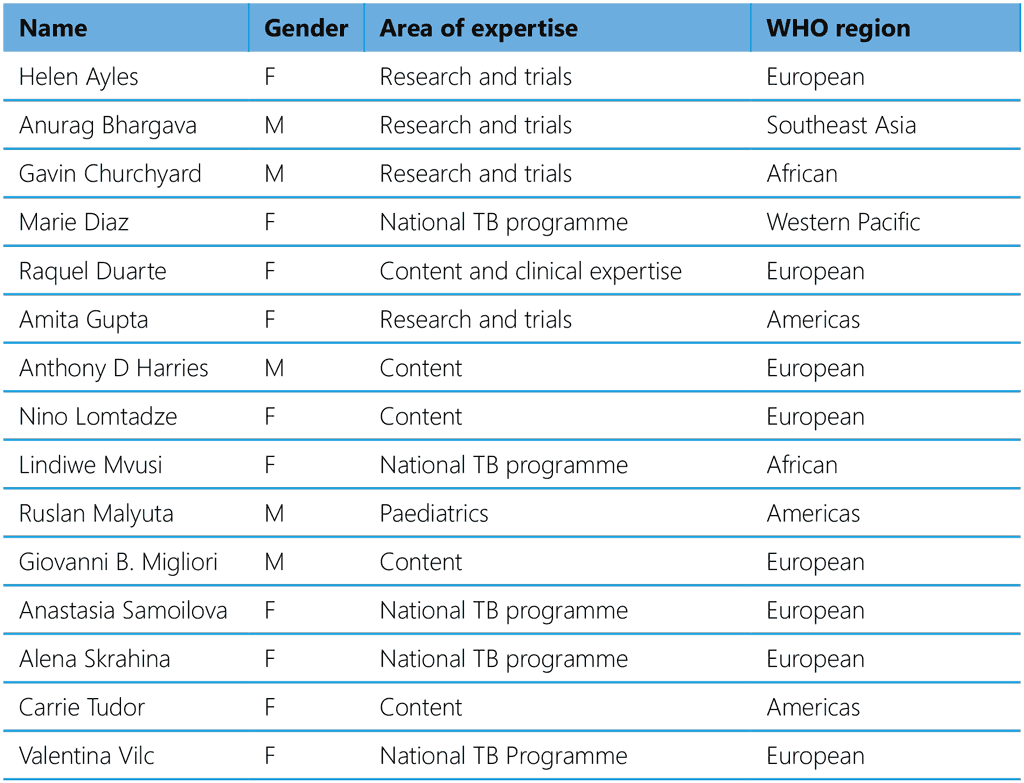
Table A2.5. External Review Group, 2023–2024
A2.7 Declarations of interests and management of potential conflict
The members of the GDG and ERG for the second edition of the TPT guidelines completed a WHO declaration of interests form in 2023. All the declarations were evaluated by the WHO Guideline Steering Group for any financial conflict of interest that might warrant exclusion from membership or from certain discussions of the GDG. The completed forms were summarized and presented to all GDG members on the first day of the meeting, at which time the members were requested to update their declarations. Intellectual conflict of interest was not considered a motive for exclusion from the GDG, as expertise on a topic was considered an important criterion for selection, and the diversity and representation in the Group was wide enough to balance any individual member’s intellectual interest.
Guideline Development Group
The following GDG members declared no interests that could conflict with the objectives of the guidelines: Mênonli Adjobimey, Ana Ciobanu, Naira Khachataryan, Amir Khan, Blessina Kumar, Natalia Litvinenko, Charisse Malbacias, Nasehi Mashid, Alberto Matteelli, Norbert Ndjeka (chair), Stavia Turyahabwe, Paran Winami.
The following GDG members declared interests that were judged not to conflict with the objectives of the meeting:
Rolando Cedillos declared consultancy payment of US$ 3000 from the Pan American Health Organization in 2022.
Alexander Kay declared ongoing supplies of discounted Xpert MTB/RIF cartridges to Baylor Children’s Foundation in Eswatini from Cepheid for a value of about US$ 2000. He also declared current funding from the US Centers for Disease Control and Prevention to Baylor College of Medicine for about US$ 5 million for a study on support for adherence to TPT with 3HP vs 6H.
Senia Rosales Klintz reported employment by the European Centre for Disease Prevention and Control.
Nicole Salazar-Austin reported research support equivalent to 75% of her salary from the US National Institutes of Health in 2019–2023, which was unrelated to drug-resistant TB treatment or prevention; and consulting for Rutgers Global TB Institute in 2022 (value US$ 5000) for the CHIP-TB project.
Susan Swindells reported current travel support of about US$ 2000 from the US National Institutes of Health and research support until 2022 (about US$ 40 000 salary support and US$ 4000 travel support) for her role as protocol chair of the BRIEF-TB trial on 1HP and to serve as a member of the NIH Adult & Adolescent Antiretroviral Treatment Guidelines Panel, TB section. She also reported research support to her institution from ViiV Healthcare up to 2022 (US$ 10 000 in salary support).
Lawrence Mbuagbaw, the guideline methodologist, reported support from Janssen Pharmaceuticals in 2018–2020 for analysing data on use of bedaquiline for treatment of MDR-TB in South Africa (US$ 150 000).
The following GDG member declared interests that were judged to conflict with the objectives of the meeting and was recused from the meeting: Hoa Binh Nguyen reported being a team member of the V-QUIN trial and receiving payment of about US$ 100 per month from the Woolcock Institute in Australia for this work.
External Review Group
The following ERG members declared no interests that could conflict with the objectives of the guidelines: Anurag Bhargava, Marie Diaz, Anthony D. Harries, Nino Lomtadze, Lindiwe Mvusi, Ruslan Malyuta, Giovanni B. Migliori, Anastasia Samoilova, Alena Skrahina, Carrie Tudor and Valentina Vilc.
The following ERG members declared interests that were judged not to conflict with the policy of WHO or the objectives of the meeting:
Helen Ayles reported in-kind support to her research group of test kits for the diagnosis of TBI from BD Biosensor (valued at about US$ 5000), Serum Institute of India (valued at about US$ 2000) and Qiagen (valued at about US$ 2000). She also declared support to her research group from a Stop TB Partnership TB Reach grant for scaling up of TPT in conjunction with testing for TBI (US$ 699 734).
Gavin Churchyard reported research support from Sanofi to his employer, Aurum Institute, as donated rifapentine and isoniazid for the WHIP3TB trial (valued at about US$ 350 000). He declared participation in a Sanofi advisory board on rifapentine for TPT, without travel support or payment. In addition, he reported a grant from USAID via KNCV/Challenge TB grant for the WHIP3TB trial (about US$ 14.2 million). He further reported a donation of rifapentine from Lupin Ltd to IMPAACT4TB studies (valued at US$ 300 000) and an honorarium from Janssen Pharmaceuticals for participating in an advisory board for developing long-acting injectable for bedaquiline (US$ 1100).
Amita Gupta reported research grants to her university from US National Institutes of Health, UNITAID, the US Centers for Disease Control and Prevention, the US Agency for International Development and Wyncote Foundation (unspecified amounts).
Raquel Duarte reported the following grants: 2021 (current) UNITE4TB: Academia and Industry innovation and treatment for Tuberculosis. (H2020 UNITE4TB 101007873) [1 June 2021–31 May 2028] [national principal investigator]; 2019 (current) – EUSAT-RCS: European–Latin American TB Research Collaboration Network (H2020 EUSAT-RCS 823890) [1 April 2019–18 March 2024] [national principal investigator]; 2018–2022, UrbanTB: from symptoms to diagnosis of urban tuberculosis considering individual and contextual factors. What are the determinants and critical points of this delay’s pathway? (FCT POCI-01–0145-FEDER-031346, PTDC/SAL-PUB/31346/2017) [1 October 2018–30 September 2022] [co-principal investigator]. In addition, she has been working as a TB consultant for the Portuguese national and regional TB programme and is also a Member of the TB Disease Network Coordination Committee of the European Centre for Disease Prevention and Control.
Evidence reviewers
The evidence reviewers undertook data collection and summarization and provided the estimates for the evidence summaries but did not participate in formulating the recommendations for policy.
The following evidence reviewers declared no interests that could conflict with the objectives of the guidelines: Stephanie Law and Harsimren Sidhu.
The following reviewer declared interests that were judged not to conflict with the policy of WHO or the objectives of the meeting: Richard (Dick) Menzies declared research support from the Canadian Institutes of Health Research of about CAN$ 1.1 million per year in 2015–2023. The work was not associated with TPT for MDR-TB.
For the composition and declarations of interest of the GDG and other expert groups involved in formulation of earlier recommendations cited in these WHO guidelines, see the previous guidelines and related annexes (1–3,8).
References
- WHO consolidated guidelines on tuberculosis. Module 1: Prevention – tuberculosis preventive treatment. Geneva: World Health Organization; 2020 (https://iris.who.int/bitstream/handle/10665/331170/9789240001503-eng.pdf).
- WHO consolidated guidelines on tuberculosis. Module 2: Screening – systematic screening for tuberculosis disease. Geneva: World Health Organization; 2021 (https://apps.who.int/iris/bitstream/handle/10665/340255/9789240022676-eng.pdf).
- WHO consolidated guidelines on tuberculosis. Module 3: Diagnosis – Tests for TB infection. Geneva: World Health Organization; 2022 (https://iris.who.int/bitstream/handle/10665/362936/9789240056084-eng.pdf).
- The V-QUIN MDR TRIAL: A randomized controlled trial of six months of daily levofloxacin for the prevention of tuberculosis among household contacts of patients with multi-drug resistant tuberculosis. Camperdown: The Australian New Zealand Clinical Trials Registry; 2016 (https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=369817).
- A phase III cluster randomized placebo-controlled trial to assess the efficacy of preventive therapy in child and adolescent contacts of MDR-TB (TB-CHAMP). Stellenbosch: Stellenbosch University Department of Paediatrics and Child Health; 2016 (https://www.isrctn.com/ISRCTN92634082).
- WHO handbook for guideline development. Second edition. Geneva: World Health Organization; 2014 (https://www.who.int/publications/i/item/9789241548960).
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE handbook for grading quality of evidence and strength of recommendations. Updated October 2013. Hamilton (Ont): McMaster University and Evidence Prime, 2024 (http://www.guidelinedevelopment.org/).
- Latent tuberculosis infection: Updated and consolidated guidelines for programmatic management (WHO/CDS/TB/2018.4). Geneva: World Health Organization; 2018 (http://apps.who.int/iris/bitstream/handle/10665/260233/9789241550239-eng.pdf).
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi:10.1136/ bmj.39489.470347.AD.
- IRIS Home. Geneva: World Health Organization; 2024 (https://iris.who.int/).
- WHO | TB Knowledge Sharing Platform (https://extranet.who.int/tbknowledge).
- WHO operational handbook on tuberculosis. Module 1: prevention – tuberculosis preventive treatment, second edition. Geneva: World Health Organization; 2024 (in press).
- Tuberculosis data. Geneva: World Health Organization; 2024 (https://www.who.int/teams/global-tuberculosis-programme/data).
 Opinião
Opinião