Links de passagem do livro para 5.2.7.1. Recommended dosages for first-line TB medicines
Table 5.3 shows the recommended dosages for first-line TB medicines for children. These dosages are applicable to all children, irrespective of the type of TB (except for TBM treated with the short intensive regimen) and HIV status. They also apply to the 12-month TBM regimen. Evidence on alternative compositions or dosages in the longer TBM regimen has not been assessed by WHO. For implications of interactions between ART and TB medicines, see Section 7.1 on TB/HIV coinfection.
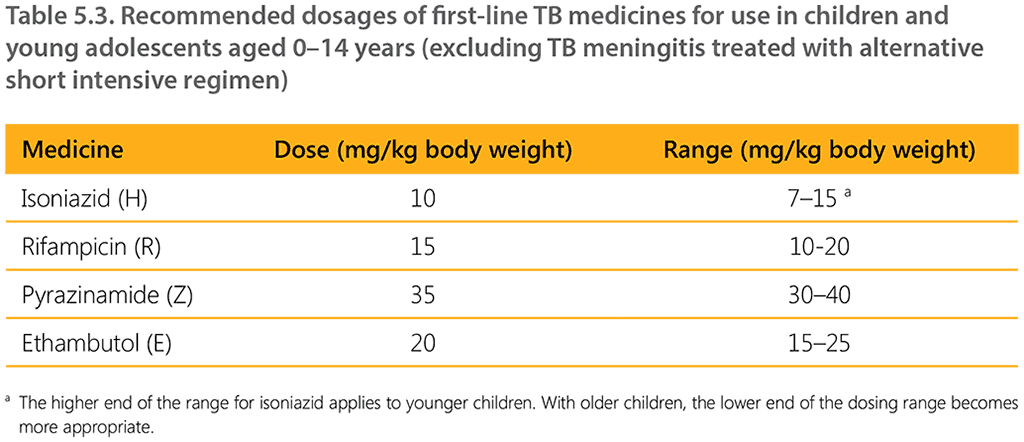
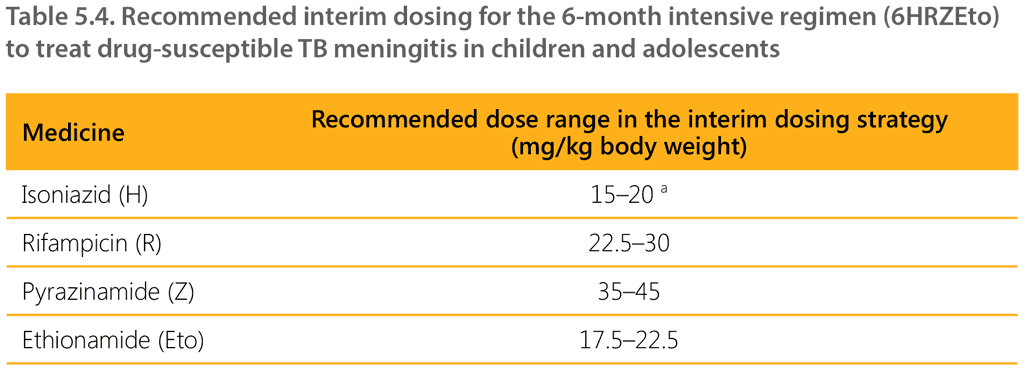
Table 5.4 shows the recommended dosages of TB medicines for children with TBM for the 6-month intensive regimen (6HRZEto). The short intensive TBM regimen uses higher doses per kilogram for isoniazid, rifampicin and pyrazinamide. Following on from the recommendation on the short intensive regimen for TBM, WHO convened an expert consultation in October 2021 to discuss how to administer the regimen, given the currently available child-friendly formulations. The experts judged that when using the available isoniazid and rifampicin (HR 50/75 mg) FDC in children, children would be exposed to a higher mg/kg dose of rifampicin (22.5–30 mg/kg), which was considered acceptable as part of the shorter TBM regimen. The dose of isoniazid should be maintained at 15–20 g/kg to avoid overexposure and associated potential toxicity.
a The higher end of the range for isoniazid applies to younger children. With older children, the lower end of the dosing range becomes more appropriate.
 Opinião
Opinião