Links de passagem do livro para 3.3.1.5 Molecular WHO-recommended rapid diagnostic tests for all other people living with HIV
The systematic review of the performance of an mWRD used to screen for TB among people living with HIV included 14 studies with a total of 9 209 participants (see Web Annex B, Table 16, and Web Annex C, Table 9). The Xpert MTB/RIF assay was the primary mWRD used in these studies. The prevalence of TB in the studies ranged from 1% to 26%. The average TB prevalence among participants attending outpatient facilities was 8.6%.
Using an mWRD alone was found to have sensitivity of 0.69 (95% CI: 0.60–0.76) and specificity of 0.98 (95% CI: 0.97–0.99) compared with using the W4SS followed by an mWRD as a diagnostic test, which had sensitivity of 0.62 (95% CI: 0.56–0.69) and specificity of 0.99 (95% CI: 0.97–0.99) (see Table 8). There were no significant differences in the accuracy of the mWRD between the different subpopulations when compared with using the W4SS followed by the mWRD.
Due to the increased sensitivity of mWRDs, but also in consideration of the likely challenges relating to access, high costs and feasibility in many countries, mWRDs are recommended as an option for screening for TB disease among all adults and adolescents living with HIV who are not medical inpatients in settings where the TB prevalence exceeds 10%. As with all screening tools, the GDG emphasized the importance in all settings of following up an mWRD screen with a diagnostic assessment to prevent the potential harm of overtreatment. In addition, due consideration should be made to prioritizing mWRDs as a diagnostic test for all people with presumptive TB before scaling up mWRD as a screening test.
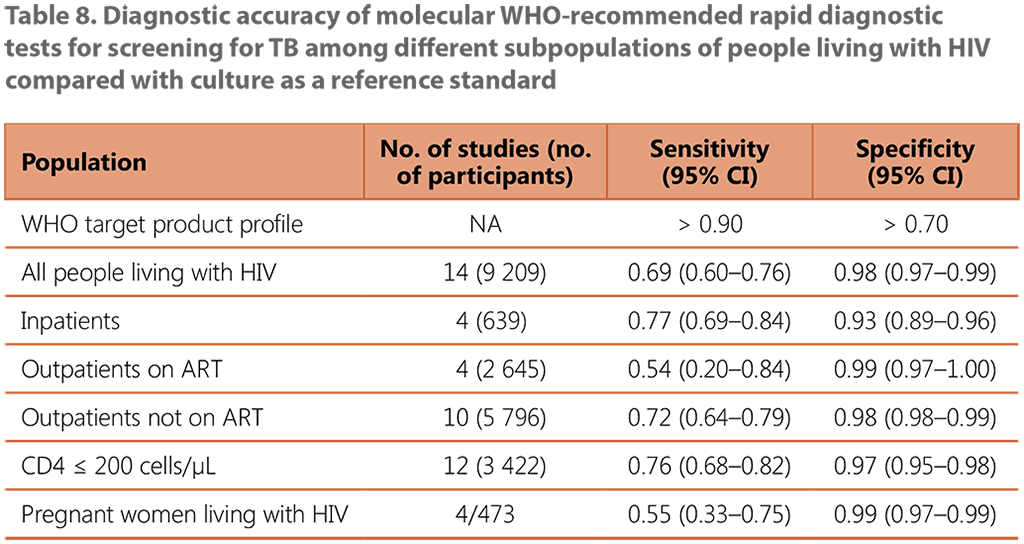
ART: antiretroviral treatment; CI: confidence interval; NA: not applicable.
 Opinião
Opinião