Links de passagem do livro para A5.2 Use of fluoroquinolones for TB preventive treatment in contacts of persons with MDR-/RR-TB: A systematic review
Harsimren Sidhu, Siobhan Carroll, Dick Menzies9
Introduction
Two randomized trials (V-QUIN and TB CHAMP) investigating safety, efficacy and tolerability of 6-month daily Lfx (6Lfx) treatment as TPT for individuals exposed to multidrug-/rifampicin-resistant tuberculosis (MDR/RR-TB) were completed in 2023. The aim of this review was to systematically review other published data from trials or observational studies on the efficacy, safety and tolerability, completion, acceptability, resource requirement, feasibility of implementation, cost-effectiveness and impact on equity of FQ regimens for TPT among all MDR/RR-TB contacts, to inform the Guideline Development Group tasked to revise the WHO TPT guidelines in December 2023. This review updated one conducted in 2016 to inform the 2018 WHO TPT guideline. The scope included studies of the efficacy and safety of other TPT regimens for MDR/RR-TB.
Methods
Research questions
- What are the efficacy, safety, tolerability, acceptability, resource requirement, feasibility, cost-effectiveness and impact on equity of Lfx (or moxifloxacin (MFX) given as TPT in people of all ages and settings exposed to MDR/RR-TB?
- What are the safety and efficacy of all other TPT drug regimens for individuals in contact with MDR/RR-TB patients?
For both objectives, searches were performed in PubMed, Embase, Turning Research Into Practice (TRIP) and the Global Health Library. For randomized trials, the Cochrane Central Register of Controlled Trials (CENTRAL) was also searched. No language restriction was set for any of the searches. Relevant studies were also identified in the reference lists of relevant studies.
Inclusion criteria
Objective 1:
- Use of FQ (Lfx/MFX) TPT for contacts of MDR/RR-TB index patients.
- At least one of the following outcomes reported: TB disease incidence, change in TB-related and all-cause mortality, adverse events, treatment completion rate, emergence of additional FQ resistance in TB strains or in the microbiome other than TB strains, resources required for implementation, impact on equity, patient and health-care worker values and acceptability of FQ-based TPT, cost-effectiveness and feasibility.
- Study designs: any longitudinal design (cohorts, case–control studies, case series, population-based observational studies), cost-effectiveness modelling and RCTs.
Objective 2:
- Must include one of the following TPT regimens: 6 or 9H, 12H, 18–36H, 3 or 4HR, 1HP, 3HP, 4R, bedaquiline, delamanid, ethambutol (EMB), ethionamide/ protionamide (ETH/PTH) or other recommended TPT regimens (not Lfx or MFX).
- Must include one of the following outcomes: TB disease incidence, prevention of disease, estimated TB-related and all-cause mortality and risk of adverse events.
- Study design: randomized control or observational studies.
Exclusion criteria
Literature reviews, abstracts, case reports, opinion articles, grey literature. For objective 2 specifically, studies that did not provide denominators to allow estimates of TB disease incidence or incidence (risk) of adverse events or had fewer than 20 participants.
Quality assessment of included studies
Two reviewers independently evaluated the design and the quality of the evidence in the included articles. Differences were resolved through discussion until a consensus was reached. For observational studies, a quality assessment questionnaire was developed to evaluate bias with items from the Newcastle–Ottawa Scale (1). The cross-sectional studies (with acceptability as the outcome) were evaluated using the AXIS tool and the one cost-effectiveness study included was evaluated using the Joanna-Briggs Institute critical appraisal tool for economic evaluations (2,3). All studies were categorized into either “high”, “medium”, or “low” risk of bias for all items on the quality assessment forms. The Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework was used to evaluate the quality of study evidence.
Fig. A5.3. PRISMA flow diagram of screening
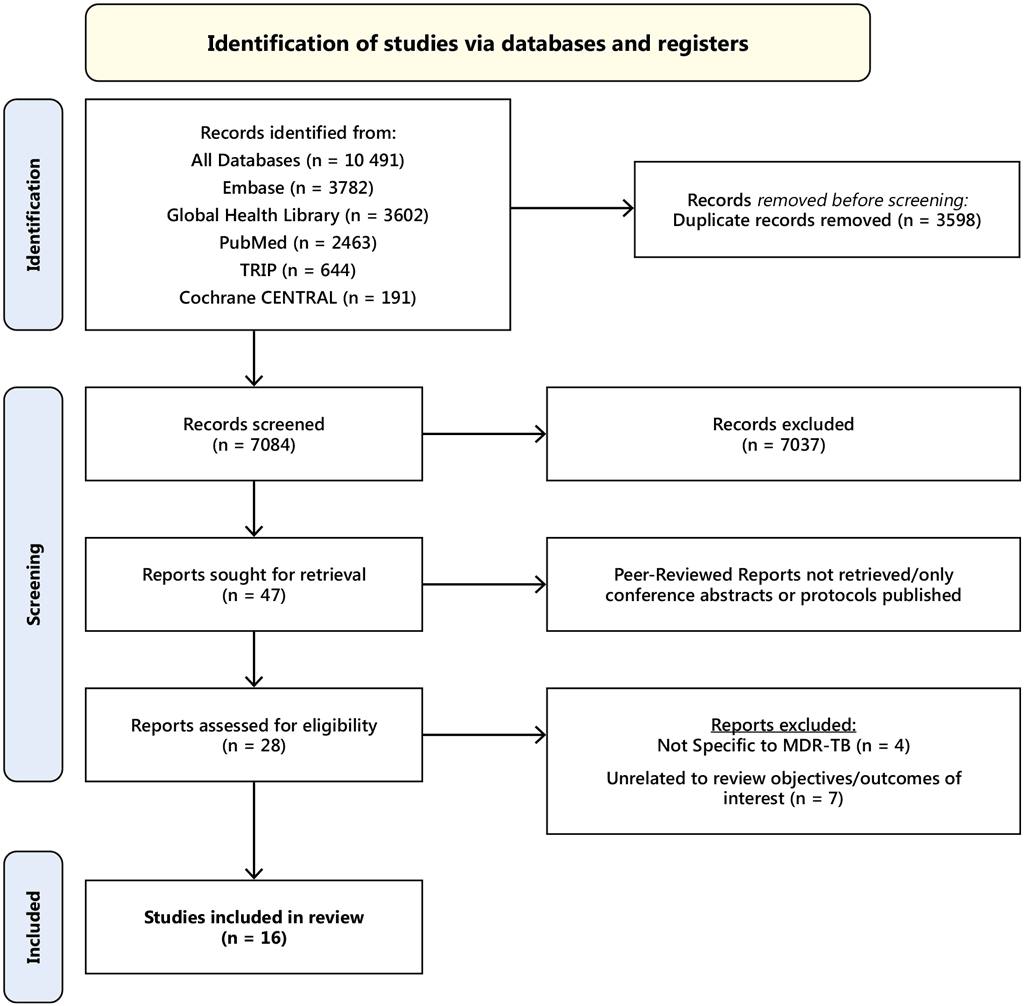
No RCTs were found. All the studies included were observational, and no studies of resource requirements, feasibility or impact on equity were found. Four of the 16 studies included efficacy: one with MFX or ofloxacin (OFX) monotherapy, two with Lfx or MFX with a companion drug (ETH, EMB or PZA and one with standard INH therapy. Five studies reported on safety outcomes, four of which were of children and adolescents and one of adult contacts. Six studies reported the acceptability of FQ-based TPT; five reported quantitative measures and one the qualitative acceptability of a novel paediatric Lfx formulation to caregivers and child contacts. One study evaluated the global cost-effectiveness of providing Lfx to HHCs < 15 years. The studies included were too disparate to allow any pooling of results or meta-analyses. Therefore, the results below reflect non-pooled findings. The protocol of this systematic review was registered on Prospero on 23 September 2023 (ID: CRD42023462793).
Results and quality of evidence
Efficacy of TPT regimens for contacts exposed to MDR-TB
Table A5.10. Observational studies evaluating the efficacy of FQ-based TPT regimens to prevent TB among HHCs of MDR-TB patients
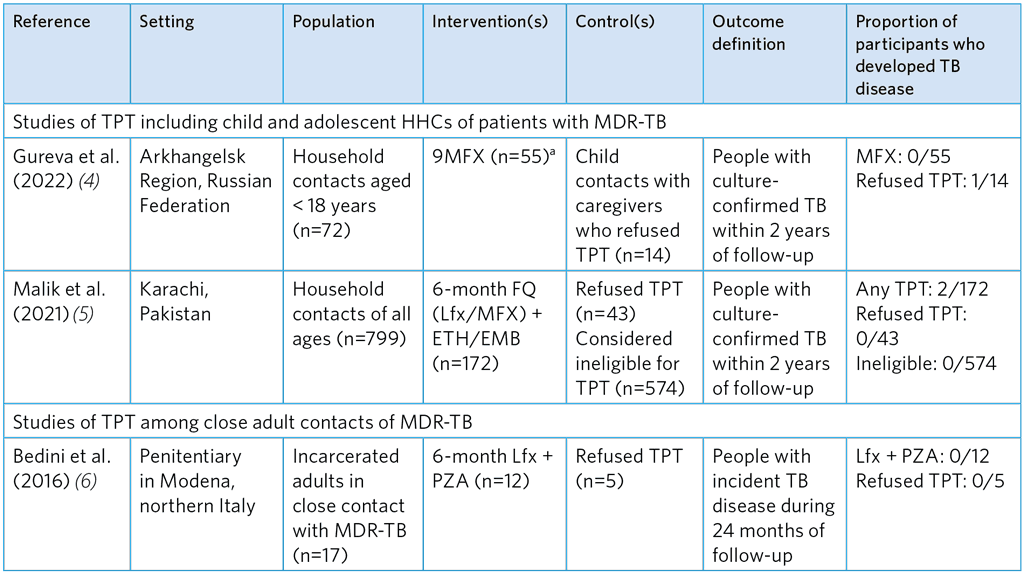
9MFX, 9 months of moxifloxacin; FQ, fluoroquinolone; Lfx; levofloxacin; MDR-TB, multidrug-resistant TB; ETH, ethionamide; EMB, ethambutol; PZA, pyrazinamide.
a Three participants were treated for 9 months with ofloxacin but are not cited here due to stronger evidence from other studies with different estimates of efficacy
Overall, the studies show that FQ-based TPT is not associated with a significant reduction of TB disease. Quality assessment suggests considerable risk of selection bias and small sample sizes, making estimates of efficacy imprecise. Gureva et al. (4) used a very small comparator group and was biased, as refusal was likely to be associated with other factors that affect health outcomes. INH was found to be effective for MDR-TB contacts in the study by Huang et al. (7) (incident TB aHR 0.19) conducted among children and adolescents < 19 years in Lima, Peru (see Table A5.11); however, potential selection bias in this study was high. The reason why the comparator group was untreated with INH is unclear but was presumably due to refusal. The mean duration of INH treatment was 115 days due to cessation TPT when multidrug resistance was confirmed, which is significantly shorter than the usual 180 days.
Table A5.11. Summary of a prospective cohort study evaluating of the efficacy of INH TPT for HHCs of MDR-TB index patients (7)
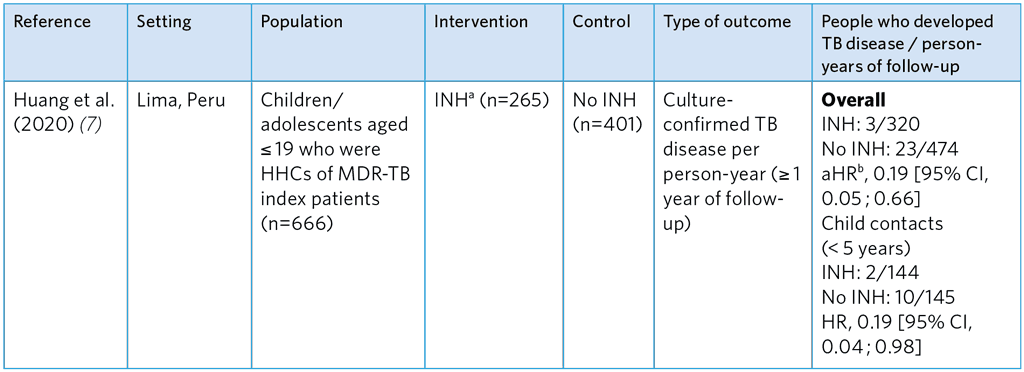
HHCs, household contacts; aHR, adjusted hazard ratio; INH, isoniazid
a Duration of treatment varied among participants, as some were told by their physicians to stop treatment after MDR-TB confirmation.
b Hazard ratio adjusted for index case age, recreational drug use, HHC, age, sex, bacillus Calmette-Guérin vaccination scar, nutritional status, being a student, TB history, household socioeconomic status and household residential district.
Safety of TPT regimens used among MDR-TB contacts
Table A5.12. Summary of studies evaluating the safety of FQ-based TPT regimens for children and adolescent (< 18)
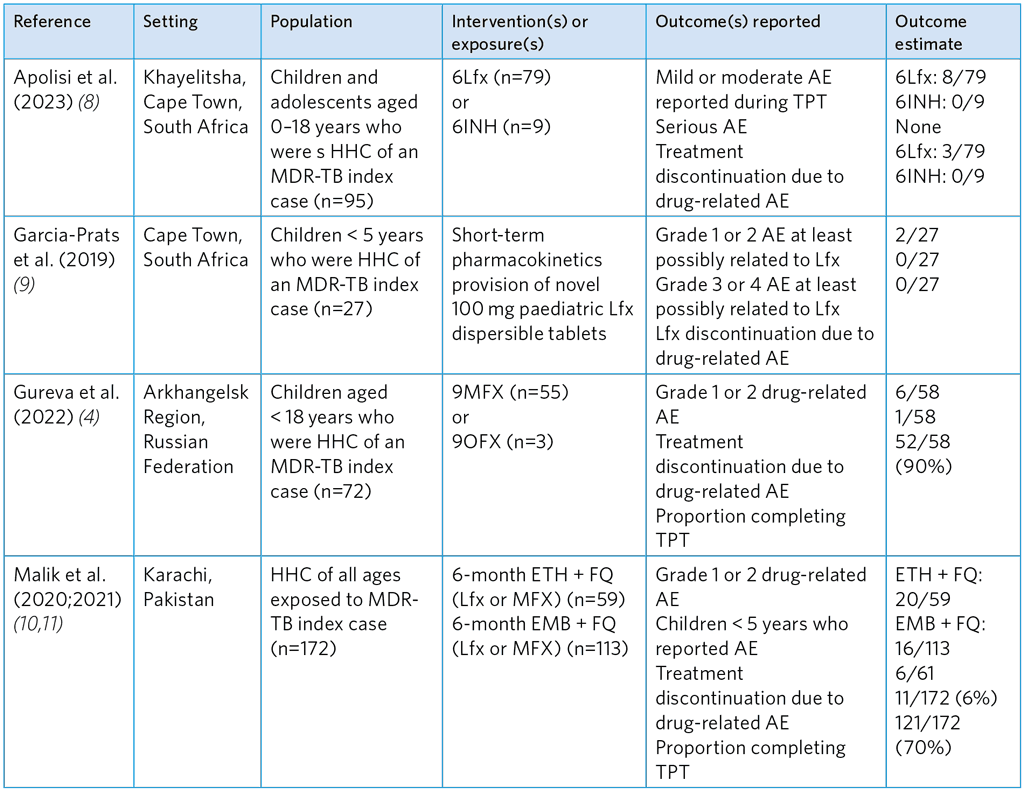
6Lfx, 6-months of levofloxacin; 6INH, 6 months of isoniazid; 9MFX, 9 months of moxifloxacin; 9OFX, 9 months of ofloxacin; HHC, household contacts
Of the five studies evaluating the safety of FQ-based TPT among exposed MDR-TB contacts, three reported AE and treatment discontinuation after FQ monotherapy with either Lfx, OFX or MFX alone (Table A5.12). No serious or grade 3/4 AE were reported in these studies and very low discontinuation of FQ treatment. The AE rates were higher in the study of Malik et al. (10), in which Lfx/MFX was given with ETH or EMB, and in the study by Bedini et al. (6) in which contacts received Lfx and PZA (Table A5.13). Malik et al. found a higher proportion of grade 1 or 2 AE with ETH than with EMB, and 11 of the 36 contacts discontinued TPT. Similarly, in the study by Bedini et al. (6), the combination of Lfx and pyrazinamide was poorly tolerated.
Table A5.13. Summary of study evaluating the safety of FQ-based TPT regimens among adult HHCs of MDR-TB index patients
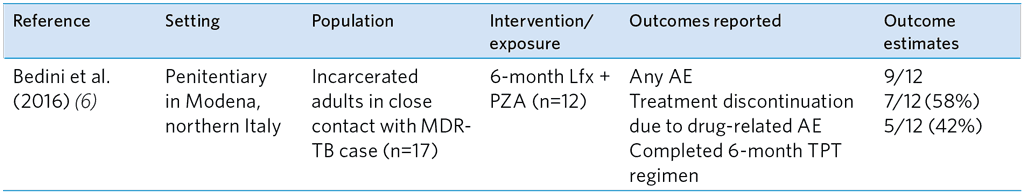
AE, adverse events; Lfx, Levofloxacin; MDR-TB, multidrug-resistant TB; PZA, Pyrazinamide; TPT, TB preventive treatment
The objective of the review was to determine the safety of FQ in MDR prevention or treatment. Data from three studies that reported AEs attributable to Lfx or MFX were retrieved. A study by Ali et al. (12) addressed acute Fridericia-corrected QT interval (QTcF) responses to experimentally administered TB drugs, including Lfx and MFX, either alone or in combination with another drug. MFX given alone resulted in only one mild (grade 1) QTcF prolongation in 32 patients, and Lfx alone resulted in QTcF prolongation in 19 patients. A pharmacokinetics study by Jin et al. (13) reported a significant association between higher Lfx concentration and increased QTc intervals; however, the QTc intervals decreased over time, and there was no significant difference from pre-treatment intervals by the end of 12 months. Treatment was not discontinued in any patient, and no patients experienced cardiac adverse events. A study conducted by Garcia-Prats et al. (14) among 70 children aged < 15 years treated for MDR-TB disease found a significant number of grade 1 AE (59/70) and grade 2 AE (11/70) that were related to Lfx. Only one child experienced a grade 3 AE, and no children experienced grade 4 AE. Treatment was not discontinued.
Acceptance, willingness and acceptability of FQ-based TPT regimens
For this review, two quantitative and one qualitative outcome were considered for acceptance (actually starting), stated willingness to start (theoretical) and acceptability according to on qualitative methods. Acceptance was defined as the proportion of eligible contacts who accepted and started TPT when offered.
Table A5.14. Summary of studies of acceptance to start FQ-based TPT among caregivers and MDR-TB HHCs
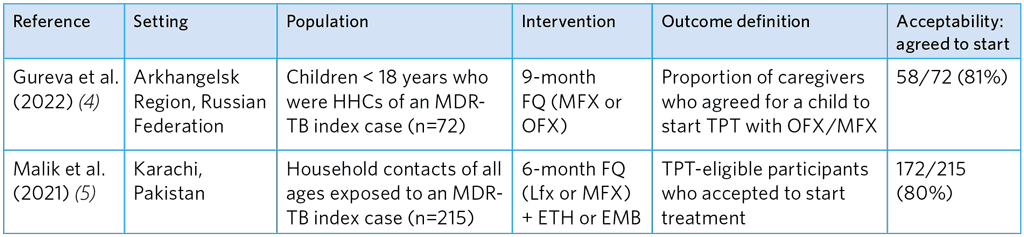
ETH, ethionamide; EMB, ethambutol; FQ, fluoroquinolone; HHC, household contacts; Lfx, levofloxacin; MFX, moxifloxacin; OFX, ofloxacin; TPT, TB preventive treatment
The reported degree of acceptance in these two studies was relatively high (Table A5.14). Gureva et al. (4) reported acceptance of 81% with MFX or OFX and Malik et al. found 80% acceptance of Lfx or MFX and a companion drug (ETH or EMB). Strong willingness was noted among adult and adolescents (Table A5.15), which, however, dropped for TPT that had potential side-effects.
Table A5.15. Studies of willingness to start hypothetical fluoroquinolone-based TPT among caregivers and MDR-TB HHCs
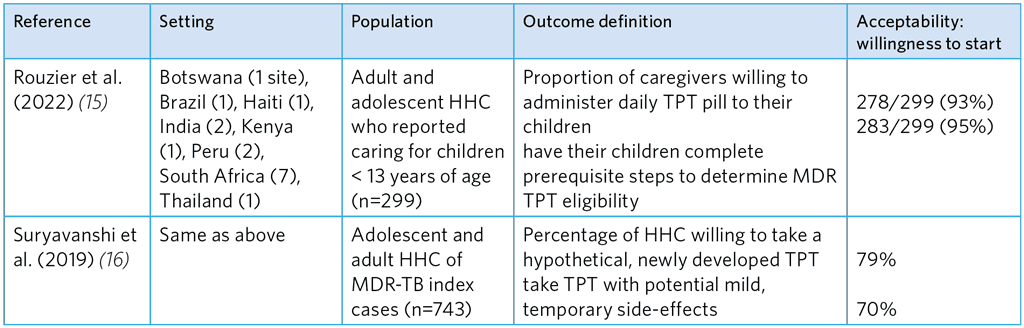
HHC, household contacts; MDR-TB, multidrug-resistant TB; TPT, TB preventive treatment
The acceptability of Lfx, i.e. willingness and ability to adhere to a TPT regimen, was addressed in two studies of a novel child-friendly Lfx formulation. Purchase et al. (17) found high acceptability among children and their caregivers; for example, 81% of caregivers found the formulation easier to prepare than the adult formulation, and 82% found the size of the tablet to be acceptable. Wademen et al. (18) also found high acceptability, although caregivers expressed concern about the financial and care burden, especially when they themselves were on treatment for MDR-TB disease.
Cost-effectiveness of TPT among children exposed to MDR-TB
The cost-effectiveness of several contact management strategies was examined by modelling in a study by Dodd et al. (19) (Table A5.16). The authors reported that provision of TPT with screening and treatment of co-prevalent TB disease was more cost-effective than detection and treatment of disease among HHCs of MDR-TB patients alone. TPT for groups at highest risk was the most cost-effective strategy, and providing TPT to all children under 15 averted most deaths and the greatest reduction in life-years lost. When the analysis was updated with efficacy estimates from the TB CHAMP and V-QUIN trials, the results were similar (unpublished data provided by J. Seddon).
Table A5.16. Summary of Dodd et al. (19) global modelling study on the cost-effectiveness of several MDR-TB HHC management scenarios for children < 15 years
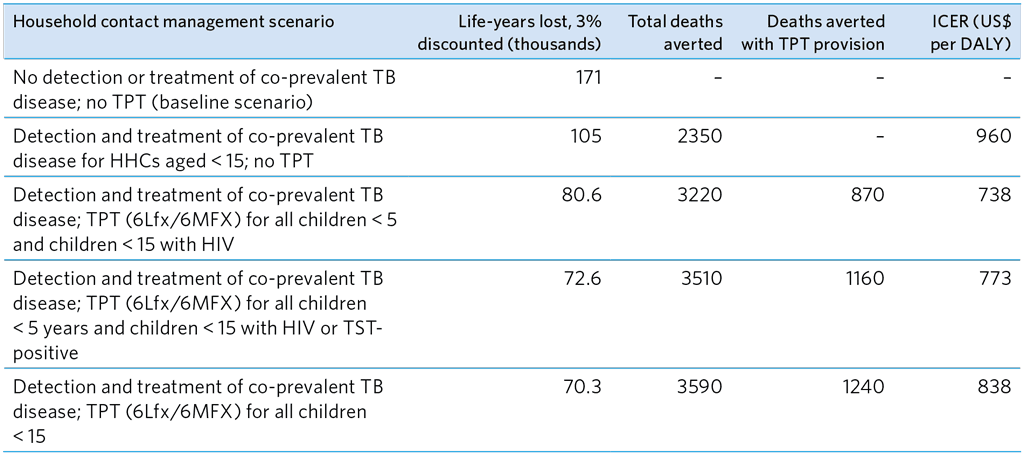
6Lfx, 6 months of levofloxacin; 6MFX, 6 months of moxifloxacin; HHC, household contacts; ICER, incremental cost-effectiveness ratio; DALY, disability-adjusted life year; TPT, TB preventive treatment; TST, tuberculin skin test
Conclusion
No randomized controlled trials that addressed the objectives of this systematic review were identified. Hence, no high-quality evidence on the efficacy of FQ-based TPT for MDR-TB contacts was found. All the observational studies identified had problems of selection bias and small samples; none suggested any significant benefit of use of FQ-based TPT to prevent development of MDR-TB disease. Although the quality of evidence was low, the results from larger observational studies suggest that FQ-based TPT is safe for use among MDR-TB contacts. No grade 3, 4 or serious adverse events related to Lfx or MFX were reported, and FQ monotherapy had high completion rates and acceptability. Mild or moderate adverse events were observed in children and adolescents. A high-quality modelling study evaluating cost-effectiveness found that targeting the highest risk groups – children < 5 or < 15 years with HIV – was the most cost-effective, but provision of FQ TPT to all contacts < 15 years would have greater impact and still be more cost-effective than detection of prevalent TB disease alone. Higher quality evidence is necessary on the efficacy of FQ-based TPT for prevention of MDR-TB disease among contacts.
References
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. ScienceOpen. 2015 (http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm, accessed 9 September 2023).
- Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016;6(12). doi:10.1136/bmjopen-2016–011458.
- Gomersall JS, Jadotte YT, Xue Y, Lockwood S, Riddle D, Preda A. Conducting systematic reviews of economic evaluations. Int J Evid Based Healthc. 2015;13(3):170–8. doi:10.1097/XEB.0000000000000063.
- Gureva T, Turkova A, Yablokova E, Smirnova P, Sveshnikova O, Zolotaya O et al. Fluoroquinolone preventive therapy for children exposed to MDR-TB. Int J Tuberc Lung Dis. 2022;26(2):171–3. doi:10.5588/ijtld.21.0443.
- Malik AA, Becerra MC, Lash TL, Cranmer LM, Omer SB, Fuad J et al. Risk factors for adverse events in household contacts prescribed preventive treatment for drug-resistant tuberculosis exposure. Clin Infect Dis. 2021;72(10):1709–15. doi:10.1093/cid/ciaa327.
- Bedini A, Garlassi E, Stentarelli C, Petrella S, Meacci M, Meccugni B et al. Multidrug-resistant tuberculosis outbreak in an Italian prison: tolerance of pyrazinamide plus LFX prophylaxis and serial interferon gamma release assays. New Microbes New Infect. 2016;12:45–51. doi:10.1016/j.nmni.2016.03.010.
- Huang CC, Becerra MC, Calderon R, Contreras C, Galea J, Grandjean L et al. Isoniazid preventive therapy in contacts of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2020;202(8):1159–68. doi:10.1164/ rccm.201908–1576OC.
- Apolisi I, Cox H, Tyeku N, Daniels J, Mathee S, Cariem R et al. Tuberculosis diagnosis and preventive monotherapy among children and adolescents exposed to rifampicin-resistant tuberculosis in the household. Open Forum Infect Dis. 2023;10(3):ofad087. doi:10.1093/ofid/ofad087.
- Garcia-Prats AJ, Draper HR, Finlayson H, Winckler J, Burger A, Fourie B et al. Clinical and cardiac safety of long-term levofloxacin in children treated for multidrug-resistant tuberculosis. Clin Infect Dis. 2018;67(11): 1777–80. doi:10.1093/cid/ciy416.
- Malik AA, Fuad J, Siddiqui S, Amanullah F, Jaswal M, Barry Z et al. Tuberculosis preventive therapy for individuals exposed to drug-resistant tuberculosis: feasibility and safety of a community-based delivery of fluoroquinolone-containing preventive regimen. Clin Infect Dis. 2020;70(9):1958–65. doi:10.1093/cid/ciz502.
- Malik AA, Gandhi NR, Lash TL, Cranmer LM, Omer SB, Ahmed JF et al. Effectiveness of preventive therapy for persons exposed at home to drug-resistant tuberculosis, Karachi, Pakistan. Emerg Infect Dis. 2021;27(3):805– 12. doi:10.3201/eid2703.203916.
- Ali AM, Radtke KK, Hesseling AC, Winckler J, Schaaf HS, Draper HR et al. QT interval prolongation with one or more QT-prolonging agents used as part of a multidrug regimen for rifampicin-resistant tuberculosis treatment: findings from two pediatric studies. Antimicrob Agents Chemother. 2023;67(7):e0144822. doi:10.1128/aac.01448–22.
- Jin Y, Benkeser D, Kipiani M, Maranchick NF, Mikiashvili L, Barbakadze K et al. The effect of anti-tuberculosis drug pharmacokinetics on QTc prolongation. Int J Antimicrob Agents. 2023;62(4):106939. doi:10.1016/j. ijantimicag.2023.106939.
- Garcia-Prats AJ, Purchase SE, Osman M, Draper HR, Schaaf HS, Wiesner L et al. Pharmacokinetics, safety, and dosing of novel pediatric levofloxacin dispersible tablets in children with multidrug-resistant tuberculosis exposure. Antimicrob Agents Chemother. 2019;63(4):e01865–18. doi:10.1128/AAC.01865–18.
- Rouzier V, Murrill M, Kim S, Naini L, Shenje J, Mitchell E et al. Caregiver willingness to give TPT to children living with drug-resistant TB patients. Int J Tuberc Lung Dis. 2022;26(10):949–55. doi:10.5588/ijtld.21.0760.
- Suryavanshi N, Murrill M, Gupta A, Hughes M, Hesseling A, Kim S et al. Willingness to take multidrug-resistant tuberculosis (MDR-TB) preventive therapy among adult and adolescent household contacts of MDR-TB index cases: an international multisite cross-sectional study. Clin Infect Dis. 2020;70(3):436–45. doi:10.1093/cid/ciz254.
- Purchase SE, Garcia-Prats AJ, De Koker P, Draper HR, Osman M, Seddon JA et al. Acceptability of a novel levofloxacin dispersible tablet formulation in young children exposed to multidrug-resistant tuberculosis. Pediatr Infect Dis J. 2019;38(6):608–10. doi:10.1097/INF.0000000000002268.
- Wademan DT, Hoddinott G, Purchase SE, Seddon JA, Hesseling AC, Garcia-Prats AJ et al. Practical and psychosocial challenges faced by caregivers influence the acceptability of multidrug-resistant tuberculosis preventive therapy for young children. PLoS One. 2022;17(7):e0268560. doi:10.1371/journal.pone.0268560.
- Dodd PJ, Mafirakureva N, Seddon JA, McQuaid CF. The global impact of HHC management for children on multidrug-resistant and rifampicin-resistant tuberculosis cases, deaths, and health-system costs in 2019: a modelling study. Lancet Glob Health. 2022;10(7):e1034-e1044. doi:10.1016/S2214–109X(22)00113–9.
9 McGill International TB Centre & WHO Collaborating Centre in TB Research, Montreal Chest Institute, and Research Institute of the McGill University Health Centref
 Opinião
Opinião