Links de passagem do livro para 7.1.7.2. Choice of antiretroviral therapy regimen
The 2021 WHO consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring provide recommendations based on rapidly evolving evidence of safety and efficacy and programmatic experience using DTG and low-dose EFV in pregnant women and people (including children and adolescents) with TB/HIV coinfection (78).
DTG is approved for use among children aged over 4 weeks and weighing more than 3 kg. A dispersible 10 mg formulation of DTG is available on the market. Among children for whom approved dosing of DTG is not available, RAL is considered an effective option and is approved for use from birth (with dose adjustments during TB treatment). RAL should be substituted with DTG as soon as it is available from 4 weeks of life.
DTG in combination with a NRTI backbone is recommended as the preferred first-line regimen for adolescents living with HIV and for infants and children with approved DTG dosing who are starting ART. EFV at low dose (400 mg) in combination with a NRTI backbone is recommended as the alternative first-line regimen for adolescents living with HIV starting ART (except in settings with pre-treatment HIV drug resistance to EFV or NVP over 10%). EFV 400 mg can be co-administered with rifampicin-containing TB treatment, with co-administration well tolerated and plasma concentrations maintained above the levels considered to be effective (181).
If DTG is not available, the preferred first-line ART regimen is a LPV/r-based regimen. RAL should be used only under special circumstances, such as in neonates. Beyond the neonatal period, infants and children should be transitioned to DTG as soon as possible.
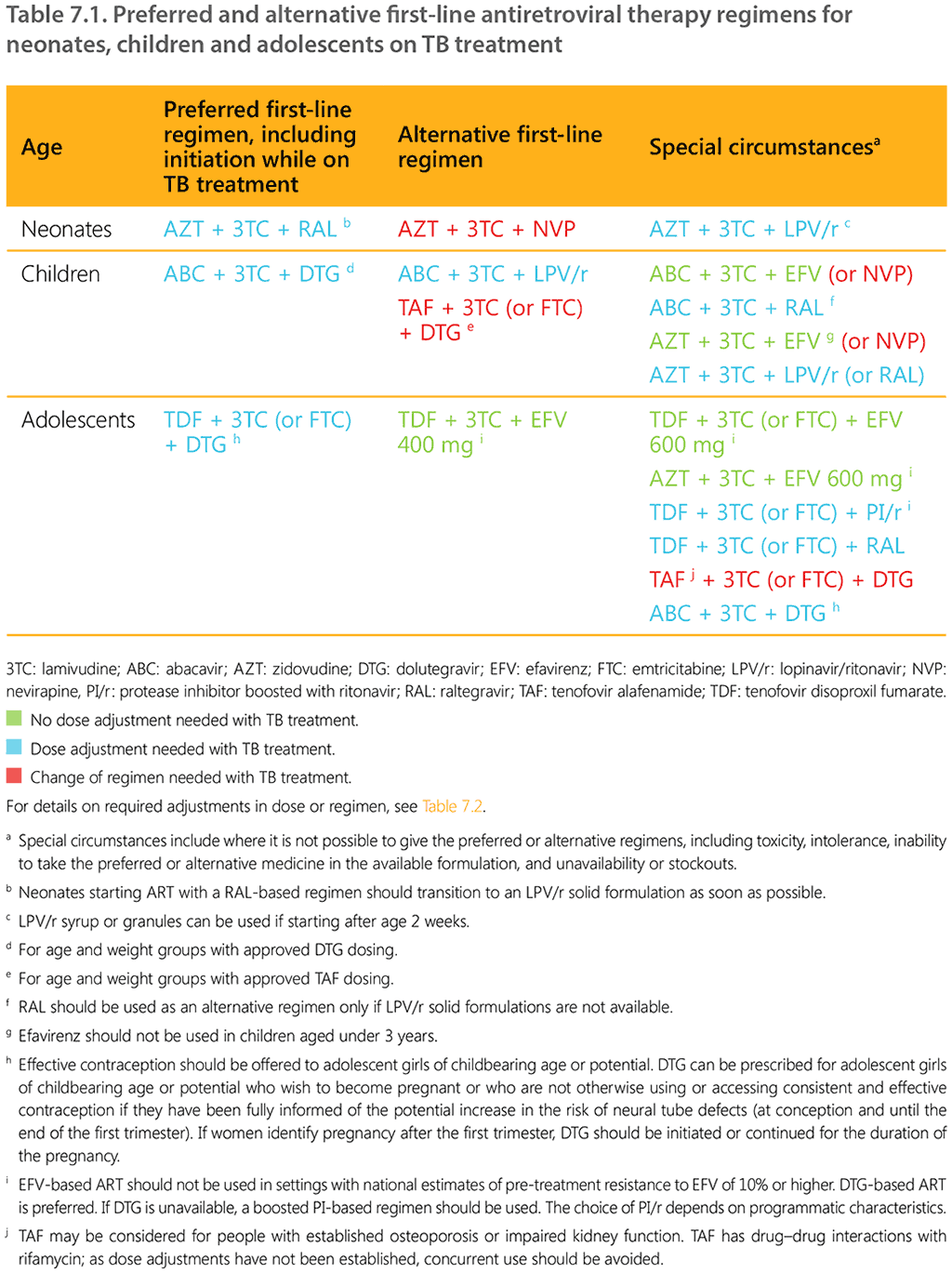
Table 7.1 summarizes the preferred and alternative first-line ART regimens for neonates, children and adolescents on TB treatment (78).
DTG in combination with an optimized NRTI backbone may be used as a preferred second-line regimen for adolescents and children living with HIV with approved DTG dosing for whom non-DTG-based regimens are failing. Boosted protease inhibitors (PIs) in combination with an optimized NRTI backbone are recommended as a preferred second-line regimen for people living with HIV for whom DTG-based regimens are failing.
 Opinião
Opinião