Links de passagem do livro para Introduction
Drug-resistant tuberculosis (DR-TB) continues to be a public health problem, taking a heavy toll on patients, communities and health care systems. Recent global estimates indicate that there were about half a million new cases of multidrug- or rifampicin-resistant TB (MDR/RR-TB) in 2018, with less than 40% of the estimated burden being notified and 32% reported to have started second-line treatment (1). Current treatment regimens for MDR/RR-TB patients are far from satisfactory. Compared with treatments for drug-susceptible forms of TB, these regimens require a longer course of treatment, a higher pill burden and the use of medicines with a higher toxicity profile; in addition, patients may develop significant adverse events and have poorer treatment outcomes. Globally, although treatment success rates have increased, almost 15% of patients with MDR/RR-TB die from the disease (1).
The Global Tuberculosis Programme of the World Health Organization (WHO/GTB) is now combining all current recommendations into one overall set of consolidated guidelines on TB. The guidelines will contain recommendations pertaining to all areas related to the programmatic management of TB (e.g. screening, preventive treatment, diagnostics, patient support, and the treatment of drug-susceptible TB and DR-TB). The consolidated guidelines will contain modules specific to each programmatic area. This current module concerns the treatment of DR-TB; it presents WHO recommendations that have been newly developed and are published here for the first time, and existing recommendations that have been published in other WHO guidelines that applied the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Structure of the document
The recommendations part of this document has seven main sections that cover aspects of the treatment of DR-TB. The aspects covered are:
- the 6-month bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) regimen for patients with MDR/RR-TB or pre-extensively drug-resistant TB (pre-XDR-TB) (Section 1);
- the 9-month all-oral regimens for MDR/RR-TB (Section 2);
- the composition and duration of longer regimens for MDR/RR-TB (Section 3);
- the treatment of rifampicin-susceptible and isoniazid-resistant TB (Hr-TB) (Section 4);
- monitoring of the patient response to MDR/RR-TB treatment (Section 5);
- antiretroviral therapy (ART) for people on MDR/RR-TB regimens (Section 6);
- the role of surgery for patients on MDR/RR-TB treatment (Section 7); and
Each section starts with the current WHO recommendations for that aspect, then gives information on the evidence used to inform that recommendation; a summary of the analyses that were carried out based on the evidence; considerations for specific subgroups; and considerations for implementation, and monitoring and evaluation. Research gaps identified for each of the sections are then presented. Web annexes provide more details on the methods, the Guideline Development Groups (GDGs), the analyses, unpublished data and statistical analysis plans. Additional information on the management of MDR/RR-TB is presented in the relevant chapter of the WHO operational handbook on tuberculosis, a separate document that is designed to aid implementation efforts (2, 3). The detailed recommendations presented here replace all previous and current WHO guidelines on the treatment of DR-TB.
Background
Effective treatment of TB, including its drug-resistant forms, relies on the use of several medicines administered in combination for an adequate duration. Significant progress has been made in recent years in identifying more efficacious, safer medicines and shorter treatment regimens. Since the 1990s, WHO has regularly evaluated evidence on the use of specific drug compositions and combinations of regimens of different durations (4–13). Historically, patients with certain drug-resistance patterns were often treated for 20 months or longer. In 2016, a standardized shorter treatment regimen (9–12 months) was recommended for patients with MDR/RR-TB strains not resistant to fluoroquinolones or second-line injectable agents, although longer regimens (18–20 months) continued to be an option for patients who were not eligible for the shorter option. Subsequent modifications to these treatment regimens led WHO to assess new evidence, which in turn resulted in revised recommendations, balancing effectiveness and harms of new regimens or modifications of recommended regimens.
Interest in reducing the duration of treatment for MDR/RR-TB has driven several initiatives in recent years to treat patients with shorter regimens under programmatic and trial conditions (14–19). When used in carefully selected patients with MDR/RR-TB who have not been previously exposed or do not have additional resistance to second-line medicines, these regimens can achieve relapse-free cure in about 80% of cases or more, even under programmatic conditions (14, 18). In 2016, on the basis of data from observational studies of the standardized shorter regimens in various countries in Africa and Asia, WHO for the first time recommended a standardized 9–12-month shorter MDR-TB regimen for eligible patients (11). In 2018, following the results of a trial – the Standard Treatment Regimen of Anti-tuberculosis Drugs for Patients with MDR-TB (STREAM) Stage 1 trial – a revised recommendation on the use of a shorter MDR-TB regimen was released, following an evidence assessment and a ranking of benefits and harms attributed to specific drugs; the revision included a recommendation to replace the injectable agent, kanamycin (or capreomycin), with amikacin (12).
Evidence of permanent effects attributed to the toxicity of injectable agents have prompted further advances in the development of new treatments such as shorter injectable-sparing regimens. In particular, South Africa’s Department of Health shared with WHO observational data on an all-oral bedaquiline-containing shorter regimen of 9 months duration. That regimen was reviewed and has been recommended by WHO since 2019, with the following combination of medicines: bedaquiline (used for 6 months), in combination with levofloxacin/moxifloxacin, ethionamide, ethambutol, isoniazid (high-dose), pyrazinamide and clofazimine for 4 months (with the possibility of extending to 6 months if the patient remains sputum smear positive at the end of 4 months); followed by 5 months of treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide (4–6 Bdq[6]-Lfx[Mfx]-Eto-E-Z-Hh-Cfz / 5 Lfx[Mfx]-Cfz-Z-E).
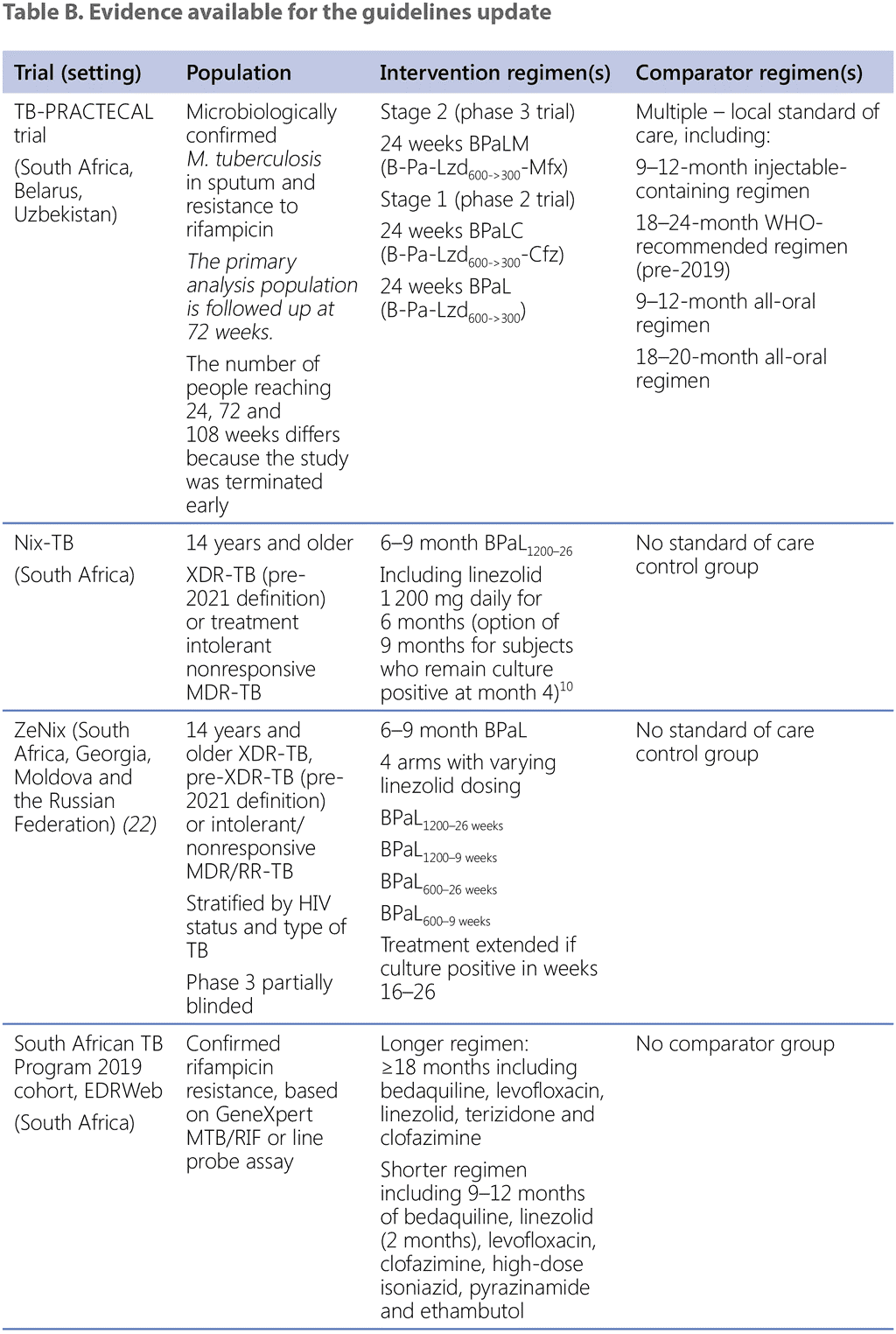
The pressing need for more effective treatment regimens for patients with extensive drug resistance, including fluoroquinolone resistance and more extensive drug-resistance profiles, has been the driver for several studies and initiatives to test more effective and novel treatment regimens, including newer and repurposed medicines. One of the first studies was the Nix-TB study, conducted by the TB Alliance. The Nix-TB study was a one-arm, Phase 3, open-label observational cohort study that assessed the safety, efficacy, tolerability and pharmacokinetic properties of a 6-month bedaquiline, pretomanid and linezolid (BPaL) treatment regimen, extendable to 9 months for those who missed doses, or remained culture positive or reverted from culture negative to positive between months 4 and 6 of treatment (20). The study was conducted between 2014 and 2019 at three study sites, all in South Africa, with the first patient enrolled in April 2015. The Nix-TB study contributed evidence to WHO that was reviewed by the GDG in November 2019 and gave rise to the previous recommendation for the use of the BPaL regimen in pre-XDR-TB patients, under operational research conditions. Two randomized controlled trials (RCTs) that concluded in 2021 (TB-PRACTECAL and ZeNix) provided new evidence and prompted assessment by WHO to develop new or updated recommendations on MDR/RR-TB treatment.
Scope of the 2022 update and available evidence
This current module on DR-TB treatment provides specific recommendations on the treatment of DR-TB, including use of regimens for rifampicin-susceptible, isoniazid-resistant TB (Hr-TB), all-oral shorter regimens for MDR/RR-TB, longer regimens for MDR/RR-TB, monitoring patient response to MDR/RR-TB treatment, starting ART in patients on second-line anti-TB regimens and undertaking surgery for patients on MDR-TB treatment.
Two new recommendations that resulted from the 2022 GDG meeting convened by WHO are on the use of new 6-month regimens, dosing of linezolid and the use of modified 9-month regimens.
Access to the new evidence was achieved through close collaboration and engagement with national TB programmes (NTPs), researchers, and a not-for-profit product-development partnership (TB Alliance) investigating the effectiveness and safety of these interventions (see Web Annex 1).
Evidence provided for the GDG review on using 6-month novel regimens was from the TB-PRACTECAL trial (evidence on using the regimens BPaLM; bedaquiline, pretomanid, linezolid and clofazimine [BPaLC]; and BPaL), ZeNix trial (evidence on using the BPaL regimen with different dosing schemes for linezolid) and Nix-TB study (evidence on using the BPaL regimen). Evidence on using a new 9-month shorter regimen was from the programmatic data provided by the NTP in South Africa.
In addition, evidence was available on the other treatment regimens that were used as external comparators, to estimate the effectiveness of the intervention regimens. The evidence included data on the use of the WHO-recommended shorter all-oral bedaquiline-containing regimen (data from the programmatic implementation in South Africa) and on the WHO-recommended longer regimens (data from country programmes in Belarus, Georgia, India, Republic of Moldova, Mozambique, Papua New Guinea, the Russian Federation and Somalia); data from fieldwork in multiple countries from Médecins Sans Frontières (MSF); and cohorts from the EndTB project provided by MSF and Partners in Health.
In preparation for the guidelines update, WHO/GTB also received data from another trial – the Newer and Emerging Treatment for MDR/RR-TB (NExT) trial – which was a Phase 2–3 open-label RCT evaluating the effectiveness of an all-oral 6–9-month regimen for the treatment of MDR-TB in South Africa (21) in comparison with a local standard of care (SoC) regimen at the time. Sharing of the data by the principal investigator and colleagues at the University of Cape Town and the South African Medical Research Council is gratefully acknowledged. However, during the GDG meeting the panel decided that the data from this study could not be used to complement discussion on the population, intervention, comparator and outcome (PICO) question designed for that study, owing to early termination of the trial and variability of the components in the intervention regimen. This does not undermine the high value of the trial results, which reiterate the inferiority and significantly worse safety profile of the DR-TB regimens based on injectable medicines and fluoroquinolones (but not including new and repurposed drugs). Importantly, the trial showed that better outcomes could be achieved with a 6-month all-oral regimen than with the traditional 9-month or longer injectable-based regimens, supporting the concept of a 6-month all-oral regimen for MDR/RR-TB.
Table B describes the evidence that was generously shared by researchers and NTPs with WHO/GTB. WHO/GTB acknowledges and commends all partners, NTPs and researchers for sharing their data.
Target audience
These guidelines are primarily targeted at policy-makers in ministries of health, or managers of NTPs who formulate country-specific TB treatment guidelines or are involved in the planning of TB treatment programmes. It is expected that these updated recommendations will also be used by health professionals, including doctors, nurses and educators working in governmental and nongovernmental organizations, and by technical agencies involved in treating patients and organizing treatment services.
10 21 patients in the Nix-TB study received linezolid 600 mg per day, at the beginning of the recruitment period.
 Opinião
Opinião