Links de passagem do livro para 4.2.1 BPaLM regimen
The BPaLM regimen includes four components – bedaquiline, pretomanid, linezolid and moxifloxacin. Bedaquiline, linezolid and moxifloxacin are used in both the 9-month regimen and the longer regimens for MDR/RR-TB (Chapter 5 and Chapter 6). when initiating the regimen, it is important to ensure that patients have not had previous exposure to bedaquiline, linezolid, pretomanid or delamanid for more than 1-month duration. When exposure is greater than 1 month, these patients may still receive these regimens if resistance to the specific medicines with such exposure has been ruled out.
The components of the BPaLM regimen all possess some bactericidal activity, making them effective anti-mycobacterial drugs when used in combination. Bedaquiline is a diarylquinoline that blocks adenosine triphosphate (ATP) synthase, pretomanid is a nitroimidazole that inhibits cell wall biosynthesis, linezolid is an oxazolidinone that inhibits protein synthesis and moxifloxacin is a fluoroquinolone that inhibits the mycobacterial topoisomerases. Further information about the mechanism of action and adverse events of each drug can be found in Web Annex 1.
In the BPaLM regimen, pretomanid is administered at 200 mg once daily. Bedaquiline is dosed at 400 mg once daily for 2 weeks, then 200 mg three times per week afterwards, according to the product label. However, in the ZeNix trial, bedaquiline was administered at 200 mg daily for 8 weeks followed by 100 mg daily; this is an alternative way to dose bedaquiline, which may be more convenient for patients and health care providers because it allows for daily dosing of all drugs throughout the regimen. Linezolid dosing is 600 mg once daily and moxifloxacin 400 mg once daily.
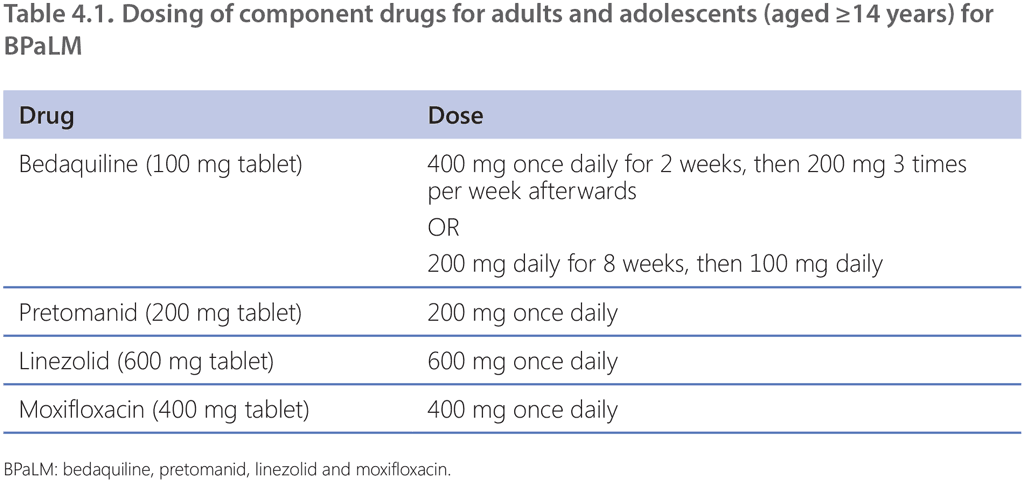
Dose modifications for bedaquiline, moxifloxacin and pretomanid are not allowed. Given the lack of evidence for the use of other fluoroquinolones, the GDG is currently unable to recommend the substitution of moxifloxacin with levofloxacin.
It is preferred to continue linezolid at the full dose for the entire duration; however, the dose of linezolid can be reduced to 300 mg or can be discontinued (and restarted when possible) if there is significant toxicity (depending on the severity of specific adverse events or serious adverse events) associated with linezolid, including optic neuritis, peripheral neuropathy or myelosuppression. Dose modification of linezolid should be avoided if possible in the first 9 weeks of therapy. Further considerations on linezolid dosing are discussed below.
 Opinião
Opinião