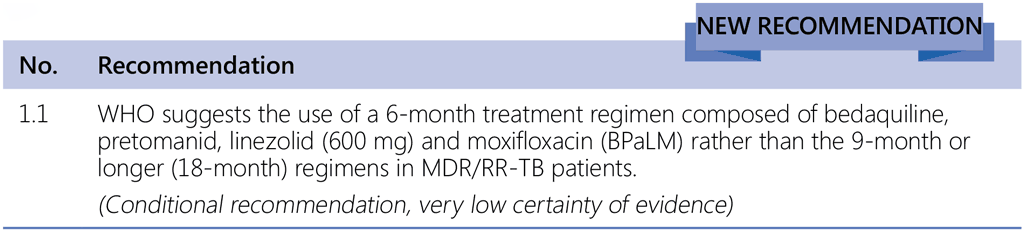
Links de passagem do livro para 1.1 Recommendations
Remarks
- Drug susceptibility testing (DST) for fluoroquinolones is strongly encouraged in people with MDR/RR-TB, and although it should not delay initiation of the BPaLM, results of the test should guide the decision on whether moxifloxacin can be retained or should be dropped from the regimen – in cases of documented resistance to fluoroquinolones, BPaL without moxifloxacin would be initiated or continued.
- This recommendation applies to the following:
- People with MDR/RR-TB or with MDR/RR-TB and resistance to fluoroquinolones (pre-XDR-TB).
- People with confirmed pulmonary TB and all forms of extrapulmonary TB except for TB involving the CNS, osteoarticular and disseminated (miliary) TB.11
- Adults and adolescents aged 14 years and older.
- All people regardless of HIV status.
- Patients with less than 1-month previous exposure to bedaquiline, linezolid, pretomanid or delamanid. When exposure is greater than 1 month, these patients may still receive these regimens if resistance to the specific medicines with such exposure has been ruled out.
- This recommendation does not apply to pregnant and breastfeeding women owing to limited evidence on the safety of pretomanid.12
- The recommended dose of linezolid is 600 mg once daily, both for the BPaLM and the BPaL regimen.13
Rationale
The rationale for this recommendation is based on the evidence and considerations described in detail in the following two subsections. Briefly, data from an RCT (stage 2 of TB-PRACTECAL, corresponds to a phase 3 trial) showed much improved treatment success rates with the BPaLM regimen (89%) of 6 months duration compared with the current SoC regimens (52%), as well as lower levels of treatment failure, death and loss to follow-up. Data from two trials (TB-PRACTECAL and ZeNix) suggested fewer adverse events with a linezolid dose of 600 mg while maintaining high efficacy. It was judged that implementing this regimen was probably feasible and acceptable, with cost–effectiveness and equity probably improved. The comparison of patient groups receiving this regimen with those receiving currently recommended regimens lasting 9 months or longer has favoured the 6-month BPaLM regimen, suggesting it to be the regimen of choice for eligible patient groups.
11 See subgroup considerations.
12 Data on the use of pretomanid in pregnant women are limited. Animal studies do not indicate direct or indirect harmful effects with respect to embryo-fetal development.
13 Additional details on linezolid dosing and possible dose reductions are given in the implementation considerations.
 Opinião
Opinião