Links de passagem do livro para A5.3 Assessing fluoroquinolone (levofloxacin) acceptability among contacts of MDR-TB patients: a qualitative study10
Objective: to assess the values, preferences, acceptability and feasibility of Lfx as TPT for adult HHCs of patients diagnosed with MDR-TB in five low- and middle-income countries: Georgia, India, Indonesia, South Africa and Viet Nam.
Sampling and recruitment strategy: Eligible participants who were contacts of newly diagnosed MDR-TB patients were identified in the five countries. In South Africa and Viet Nam , collaborators also recruited participants who were part of the V-QUIN and TB CHAMP trials, including participants who did and did not complete the study treatment due to adverse events. Collaborators at each site explained the project briefly to potential participants. Interviews were conducted in the presence of a skilled interpreter where required. Informed consent, written or verbal, was obtained before the interview.
Eligibility criteria
Inclusion:
- household contact of a person diagnosed with MDR-TB.
- eligible for TPT according to WHO guidelines (1).
Exclusion:
- < 18 years
- unable to provide informed consent
- unable to be interviewed in Cantonese, English, French, Mandarin or Punjabi or interpreter not available.
Methods
A trained qualitative researcher conducted one-on-one interviews with a semi-structured interview guide with the participants over telephone or online. Trained interpreters, hired by the interviewers, were present when required. The interviews lasted 30–60 min. The interviewer asked participants about their attitude, values and perspectives towards use of FQs as TPT and sought to understand the risk–benefit considerations underlying their decisions. Participants were informed of the estimates of effectiveness and side-effects from the preliminary results of the randomized trial study populations in Viet Nam (adults) and South Africa (children). They were also informed about the risks of MDR-TB disease, and the difference from TB infection, and risks and burden of MDR-TB treatment, including treatment duration, adverse events and treatment outcomes. This allowed participants to make an informed decision on whether they preferred TPT to an increased risk of developing MDR-TB disease. Interviewers at each site recorded demographic and clinical information, including age, sex, level of education, comorbidities and TB history on a patient enrolment form. Data were analysed with an inductive approach. Thematic analysis was used to identify and highlight recurring themes.
Table A5.17. Study participants (n=36)
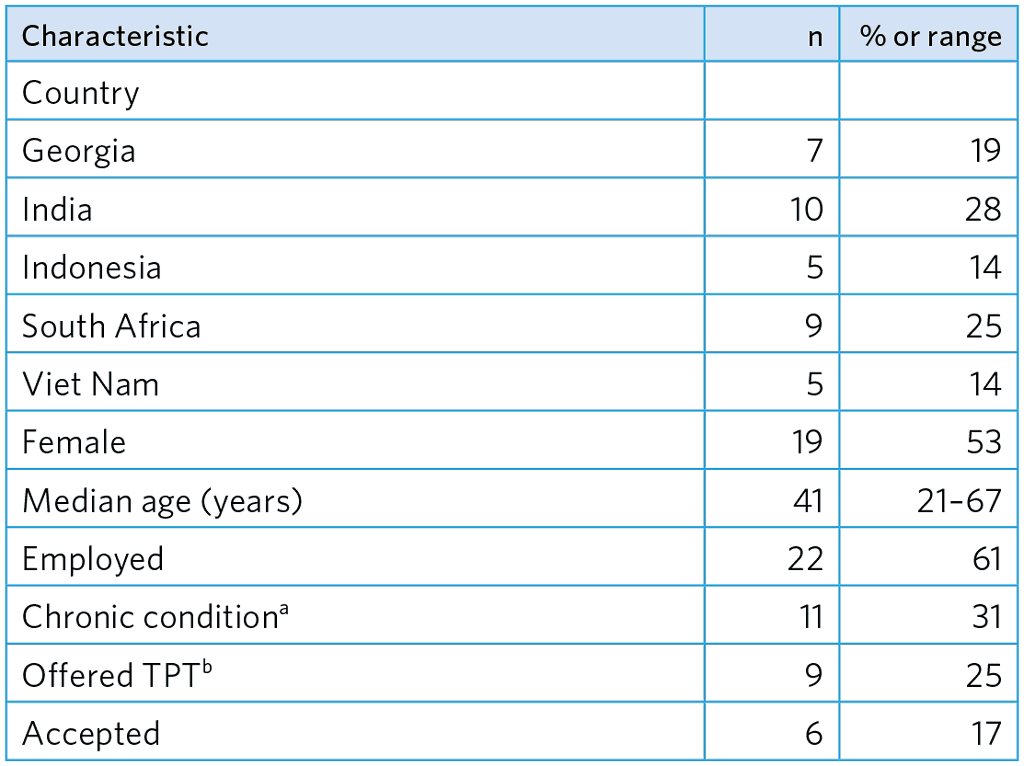
a Diabetes, cardiovascular disease, chronic gastritis, joint pain/arthritis, HIV
b 2/2 were offered and accepted TPT for DS-TB, 4/7 were offered and accepted TPT for MDR-TB (6-month Lfx).
Results
A total of 36 participants were interviewed (Table A5.17).
Acceptability of TPT for MDR-TB involved a decision on whether:
- TPT held value for them (“values”);
- TPT effectiveness, requirements and safety met their subjective thresholds (“preferences”); and
- they anticipated being able to complete the treatment successfully (“feasibility”).
The participants’ values were influenced by their sociocultural and economic contexts, as well personal and community experiences with MDR-TB. The values aligned with higher TPT acceptability included:
- belief in the importance of disease prevention, such as vaccination;
- general trust in medicines and doctors, “The doctor knows best, so whatever they give, I have to take.” (India, 45-year-old woman);
- fear of MDR-TB disease, its treatment and contagiousness, “I would feel so bad if I got MDR-TB, it will be very painful….TPT is a good thing because I have younger grandkids and we don’t know when they will catch it.” (South Africa, 50-year-old woman).
- A participant’s values could override the perceived benefits and harms of TPT. For instance, a few participants who did not value disease prevention would refuse MDR TPT, regardless of its potential effectiveness, low requirements and safety, unless it was mandatory.
Among participants who found value in TPT, acceptance depended on their subjective thresholds for treatment effectiveness, dosage and schedule and adverse drug reactions. For instance, participants would tolerate mild-to-moderate side-effects and long treatment duration, if they had a minimum level of efficacy (such as reducing the risk of disease by 50%), but not if TPT efficacy was below that threshold. Given an acceptable level of efficacy, most participants prioritized safety over treatment duration; treatment schedule was considered the least important. The final consideration of acceptability was perceived feasibility. Participants who valued TPT reflected on demands on their lives due to TPT. They considered the following as potential barriers: out-of-pocket expenses (e.g. transport); disruption due to clinical follow-up, time commitment and requirement for child-care arrangements; lack of social and financial support; and insufficient treatment counselling and education.
Overall, MDR TPT was acceptable and held a high social value among participants in the five settings. The most acceptable regimen would have high effectiveness in preventing MDR-TB, mild toxicity, little interference with daily activities, low pill-burden, minimal and convenient clinical follow-ups, and low cost to the participants.
Reference
WHO operational handbook on tuberculosis. Module 1: prevention – tuberculosis preventive treatment. Geneva: World Health Organization; 2020 (https://www.who.int/publications/i/item/9789240002906).
10 Stephanie Law, Harsimren Sidhu, Dick Menzies (Research Institute, McGill University Health Centre and McGill International TB Centre, McGill University, Montreal, Quebec, Canada); Gregory J. Fox, Thu-Anh Nguyen (Woolcock Institute of Medical Research, Ha Noi, Viet Nam, The University of Sydney, Sydney, Australia); Ciara Goslett, Anneke Hesseling, Graeme Hoddinott, Nosivuyile Vanqa, Dillon Wademan (Desmond Tutu TB Centre, Stellenbosch University, Cape Town, South Africa); Hansen Herman, Rovina Ruslami (Universitas Padjadjaran, Bandung, Indonesia); Maia Kipiani (National Center for Tuberculosis and Lung Diseases, Tbilisi, Georgia); Rupak Singla (National Institute of Tuberculosis and Respiratory Diseases, New Delhi, India); Nelly Solomonia (National Center for Tuberculosis and Lung Diseases, Tbilisi, Georgia); Duy Hoang Trinh (Woolcock Institute of Medical Research, Ha Noi, Viet Nam).
 Opinião
Opinião