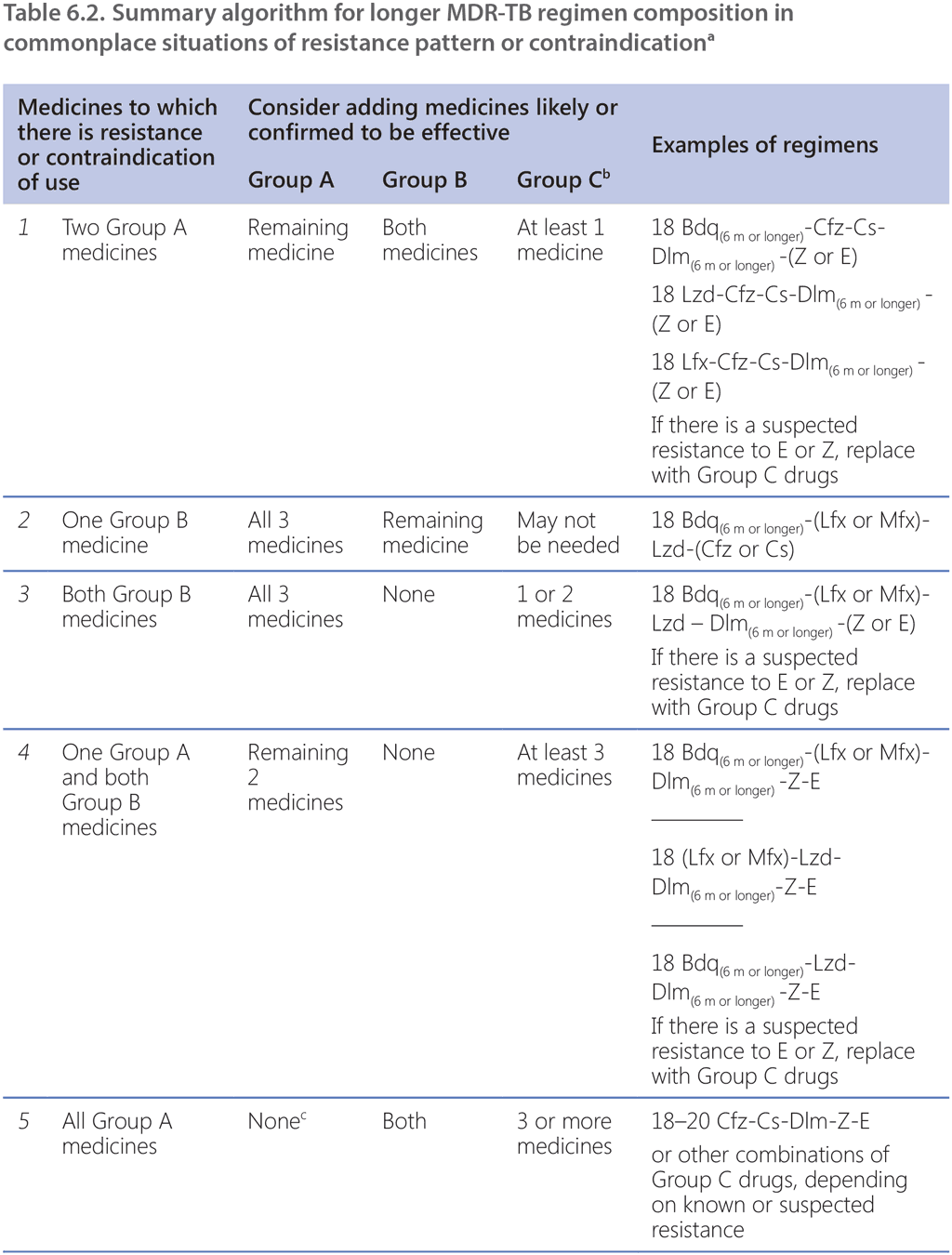
Links de passagem do livro para 6.5.2 Monitoring safety
The safety profile of some medicines used concomitantly in a long treatment regimen may present its own concerns. aDSM should be performed, as well as proper management of adverse events and prevention of complications from drug–drug interactions. The NTP should actively monitor drug safety to ensure proper patient care, to report any adverse events to the responsible drug safety authority in the country, and to inform national and global policy.
The medicines included in the selected regimen determine which monitoring tests are needed. Therefore, the monitoring schedule should consider:
- clinical assessments to identify optic and peripheral neuropathy and psychiatric disturbances;
- clinical and biochemical assessments (especially when linezolid is used for a longer period) to identify pancytopenia, lactic acidosis and peripheral neuritis including frequent eye or visual assessment and any potential drug–drug interaction (e.g. serotoninergic syndrome) and
- ECG and monitoring of electrolytes, particularly when the regimen contains multiple QT interval prolonging agents (e.g. bedaquiline, delamanid, moxifloxacin and clofazimine) – in the case of electrolyte disturbances or ECG abnormalities, more frequent monitoring should be performed.
More frequent monitoring may be advisable in specific situations; for example, in older people, People with HIV, those affected by hepatitis (caused by HBV or HCV) or diabetes, or people with moderate to severe hepatic or renal impairment.
Any adverse events during treatment should be managed immediately to relieve suffering, minimize the risk of treatment interruptions, and prevent morbidity and mortality. Details on monitoring and management of adverse events are provided in Web Annex 2 and Web Annex 3.
 Opinião
Opinião