Links de passagem do livro para 5.2.2 Linezolid variation
The linezolid variation involves initiation of bedaquiline, linezolid, levofloxacin/moxifloxacin, clofazimine, ethambutol, isoniazid (high dose) and pyrazinamide. Linezolid is only given for the first 2 months of treatment. Clinical and haematological monitoring are crucial to detect early linezolid-associated adverse events, particularly haematological events (sudden or significant drop in haemoglobin, neutrophils or platelets). After the initial 2 months, the remaining six drugs are given for another 2 months (with the possibility of extending by an additional 2 months if the patient’s sputum remains bacteriologically positive at the end of the fourth month on treatment). High-dose isoniazid is dropped after 4 or 6 months, depending on the decision to extend treatment based on smear status at month 4 of treatment. This is followed by 5 months of treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide. Bedaquiline is usually given for 6 months but could be extended to 9 months, particularly if the initial phase is extended from 4 to 6 months due to a positive sputum smear result at month 4.
The regimen is summarized as:
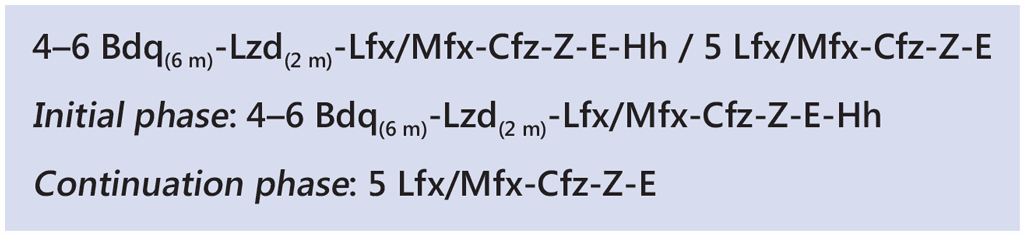
 Opinião
Opinião