Links de passagem do livro para 5.2.7.2. Dosage tables and formulations for treatment of drug-susceptible TB in children and adolescents
The use of FDC child-friendly tablets is recommended instead of separate formulations in the treatment of children with drug-susceptible TB (100). FDC tablets have advantages over single medicines as they reduce the pill burden and the likelihood of prescription errors. By reducing selective non-adherence, FDC tablets can reduce the risk of development of drug resistance.
Dispersible child-friendly FDC formulations for first-line TB medicines have been available since 2015. They can be dispersed in water to achieve an appropriate dose offering the opportunity to simplify and improve treatment (88, 100, 101). Child-friendly FDCs are WHO-prequalified. They are not new medicines but are improved formulations of medicines recommended and used for first-line treatment of TB.
Table 5.5 shows the recommended dosages by weight band (except for the short intensive TBM regimen). Note that dosages should be adjusted based on weight changes during TB treatment (see Section 5.2.12).
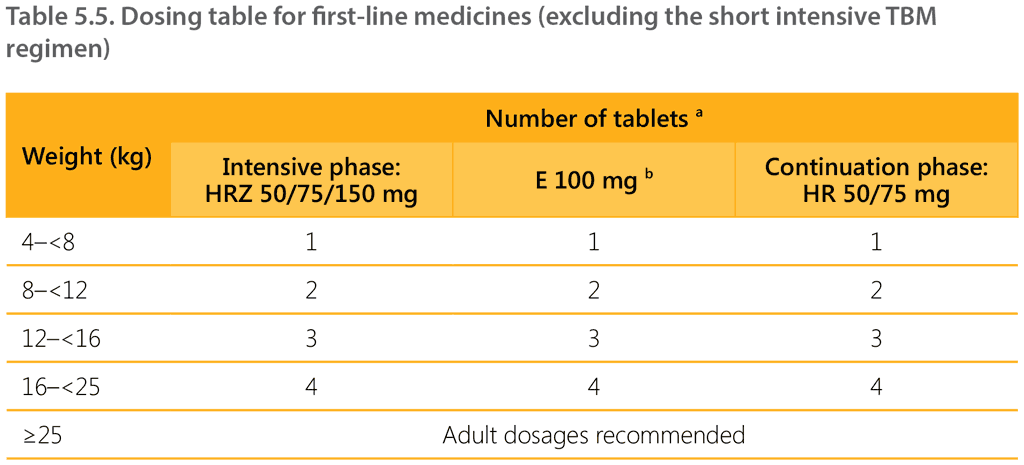
Presentation of weight bands has been updated – for example, the 4–7 kg weight band is now written as 4–<8 kg. A child weighing 7.9 kg is dosed as per the 4-<8 kg weight band, a child weighing 8 kg is dosed as per the 8-<12 kg weight band.
a Ideally, tablets should be dissolved in 50 mL water. The child should consume the complete fluid within 10 minutes of dissolving the tablets. If the child cannot consume the full amount, the tablets can be dissolved in a smaller amount of liquid.
b Ethambutol (preferably using a dispersible tablet) should be added in the intensive phase for children with extensive disease, or if living with HIV, or if living in a setting where the prevalence of HIV or of isoniazid resistance is high.
 Opinião
Opinião