Book traversal links for 3.5 Steps and processes for implementing a new diagnostic test
As an initial step in implementing a new diagnostic test, countries should review WHO policies, guidance and reports, as well as any available implementation guide from WHO, the GLI, the Foundation for Innovative New Diagnostics (FIND) and implementing partners. Particular attention should be paid to WHO policies and recommendations for the use of the test, the test’s limitations and the interpretation of test results.
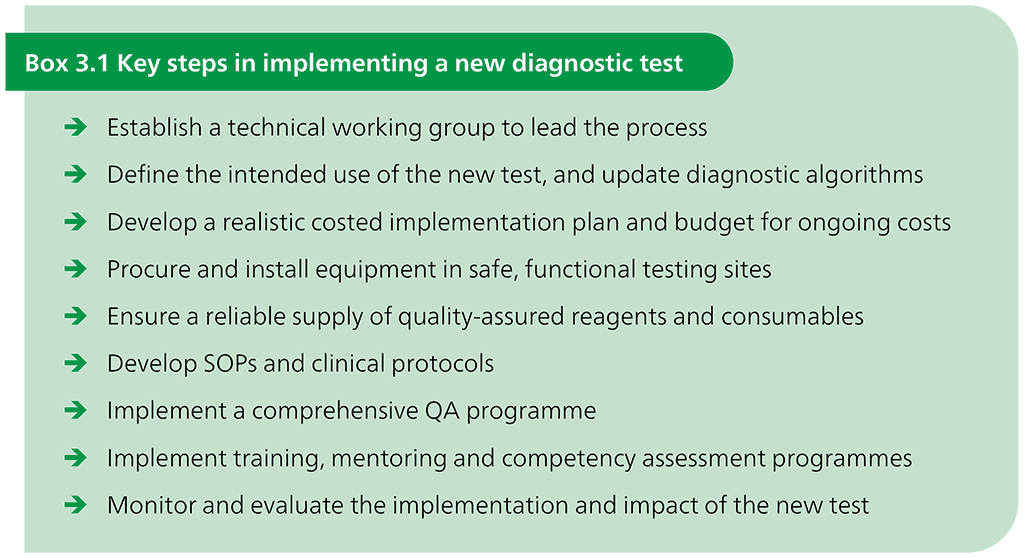
The key steps in implementing a new test are listed in Box 3.1. Critical early steps include defining the intended use of the new test, developing a costed implementation plan, building the infrastructure (instruments and facilities) and developing the human resources needed for the new test.
As described in Annex 3 and in The use of next-generation sequencing technologies for the detection of mutations associated with drug resistance in Mycobacterium tuberculosis complex: technical guide (30), implementation of targeted NGS tests follows the same basic steps described below, with some additional considerations (e.g. cost of the equipment, complexity of the assay, and need for highly skilled laboratory workers and bioinformatics resources).
Within Section 3.5, the key steps for implementing a diagnostic test are organized into 10 main areas:
- Area 1 – Policies, budgeting and planning (Section 3.5.1)
- Area 2 – Regulatory issues (Section 3.5.2)
- Area 3 – Equipment (Section 3.5.3)
- Area 4 – Supply chain (Section 3.5.4)
- Area 5 – Procedures (Section 3.5.5)
- Area 6 – Digital data (Section 3.5.6)
- Area 7 – Quality assurance, control and assessment (Section 3.5.7)
- Area 8 – Recording and reporting (Section 3.5.8)
- Area 9 – Human resource training and competency assessment (Section 3.5.9)
- Area 10 – Monitoring and evaluation (Section 3.5.10).
The rest of this section discusses the steps in each of these areas.
 Feedback
Feedback