Book traversal links for 5.3.2.1. Overview and approach to selecting a treatment regimen
This section covers the standardized shorter all-oral bedaquiline-containing regimen and individualized regimens for children and adolescents not eligible for the shorter all-oral bedaquiline-containing regimen. It also includes other important considerations, including treatment of EPTB and TB/HIV coinfection, and dosing and formulations.
The risks and benefits of each medicine should be considered carefully while designing a regimen. None of the available TB medicines is contraindicated in children. The medicines are generally well tolerated, with some exceptions: injectable agents (aminoglycosides) are associated with hearing loss, which can be difficult to monitor in young children and can have a devastating effect on cognitive and language development, education and socialization (111, 112, 114, 115).
When a decision has been made to treat a child for MDR/RR-TB, two main regimens are available. Current WHO guidelines recommend prioritizing the standardized shorter all-oral bedaquilinecontaining regimen for people with MDR/RR-TB. For people not eligible for this regimen, an individualized longer regimen composed of medicines in priority groupings A, B and C should be constructed. Current WHO drug groupings are shown in Table 5.11.
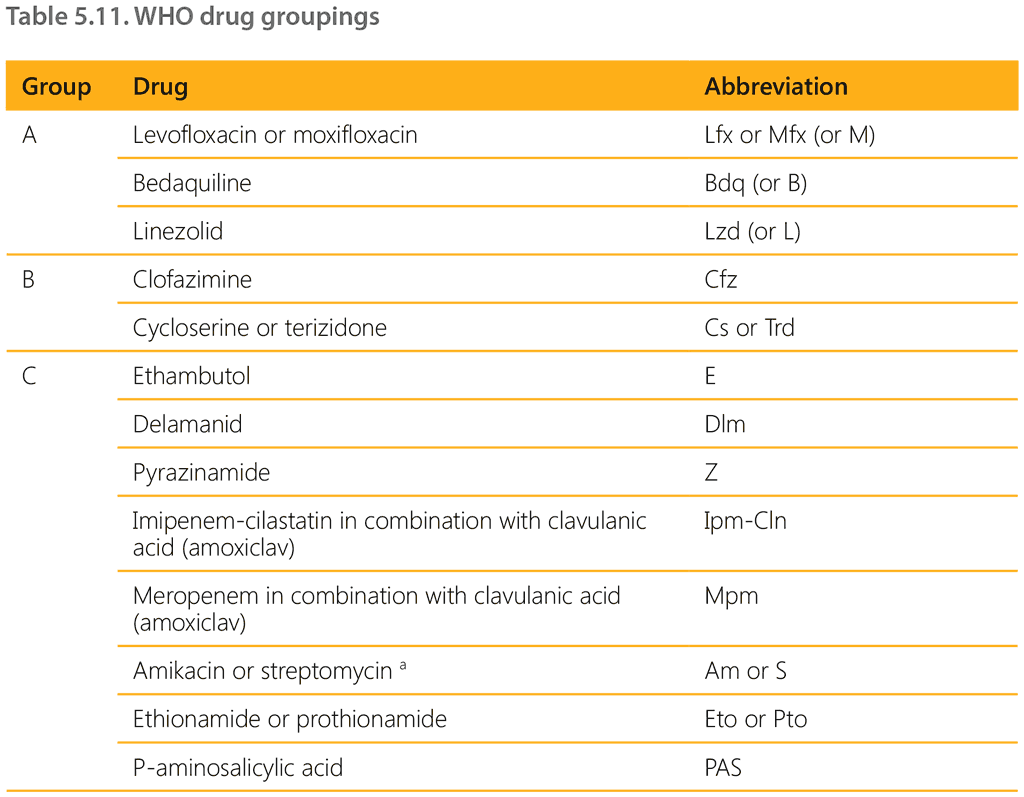
a Amikacin and streptomycin are to be considered only in adolescents aged over 18 years and only if DST results confirm susceptibility, and if high-quality audiometry monitoring for hearing loss can be ensured. Streptomycin is to be considered only if amikacin cannot be used (i.e. is unavailable or there is documented resistance) and if DST results confirm susceptibility (i.e. resistance to streptomycin is not detectable with second-line molecular LPAs and phenotypic DST is required). Kanamycin and capreomycin are no longer recommended for use in MDR-TB regimens.
The 2021 guideline development group reviewed evidence (mainly pharmacokinetic and safety data) on the use of bedaquiline in children aged under 6 years and delamanid in children aged under 3 years. The new recommendations expand the age indications for both bedaquiline (as part of shorter and longer regimens) and delamanid (as part of longer regimens) to children of all ages. These new recommendations make it possible to build all-oral treatment regimens for all children
with MDR/RR-TB.
WHO recommendations on treatment of MDR/RR-TB in children and adolescents are given in Box 5.14.


 Feedback
Feedback