Book traversal links for 5.2.4.1. Assessing eligibility for the 4-month regimen
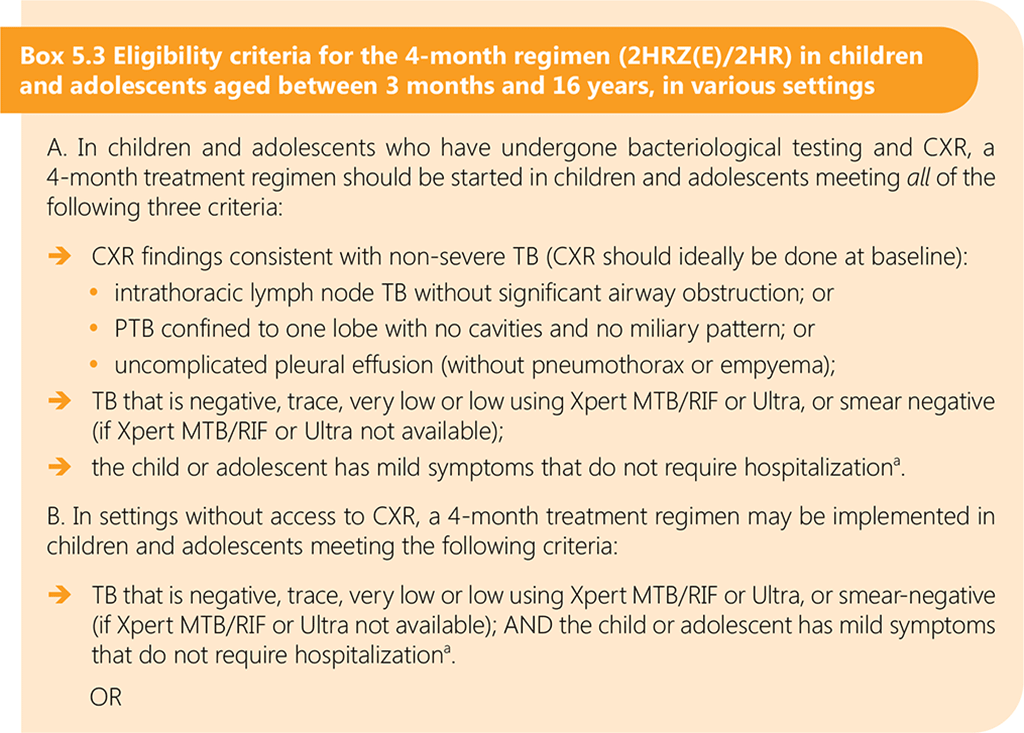
Non-severe TB is defined as peripheral lymph node TB, intrathoracic lymph node TB without airway obstruction, uncomplicated TB pleural effusion or paucibacillary, non-cavitary disease confined to one lobe of the lungs and without a miliary pattern.
As CXR is an important tool to assess severity of disease in children and adolescents, NTPs are encouraged to scale up access to good-quality CXR and provide training to health care providers in its interpretation. Out-of-pocket expenses for CXR pose a potential barrier to the diagnosis of TB and access to the shorter regimen for eligible children and young adolescents.
In the SHINE trial, children who were Xpert MTB/RIF-positive but sputum smear-negative were eligible for inclusion. The 85 children (7%) who were Xpert MTB/RIF-positive (45 in the 4-month arm, 40 in the 6-month arm) had very low or low semiquantitative Xpert MTB/RIF results (86). Therefore, in the eligibility criteria for the 4-month regimen, Xpert MTB/RIF or Ultra trace, very low or low semiquantitative results are included. If smear microscopy is used for bacteriological confirmation, the child should have a negative smear result to be eligible for the 4-month regimen.
Children with SAM, infants aged under 3 months, and children treated for TB in the past 2 years are not eligible for the 4-month regimen and should be treated with the 6-month regimen (see Section 5.2.5).
Box 5.3 provides eligibility criteria for the 4-month regimen for use in different settings, including those without access to CXR and bacteriological testing.
Availability of medicines and child-friendly formulations are other important considerations when selecting a treatment regimen.

 Feedback
Feedback