Book traversal links for Annex 2. Tuberculin skin testing: administration, reading and interpretation
This annex provides information on administering, reading and interpreting tuberculin skin tests (TSTs).
A TST is the intradermal injection of a combination of mycobacterial antigens that elicit a delayed-type hypersensitivity immune response, represented by induration, which can be measured in millimetres.
The standard method of identifying people infected with Mycobacterium tuberculosis is the TST using the Mantoux method. Multiple puncture tests should not be used as these tests are unreliable (because the amount of tuberculin injected intradermally cannot be precisely controlled). This annex describes the procedure for a TST using 5 tuberculin units (TU) of tuberculin purified protein derivative (PPD)-S. (Alternatively, 2 TU of tuberculin PPD RT 23 can be used).
Administration
- Locate and clean the injection site 5–10 cm below the elbow joint:
- Place the forearm palm-up on a firm, well-lit surface.
- Select a smooth area of skin (e.g. free from scars, sores and veins) for placing the TST.
- Clean the area with an alcohol swab.
- Prepare the syringe:
- Tuberculin vials are multidose vials (of 10 or 50 doses). The vials should be stored at 2–8 °C without exposure to direct sunlight. The vial can be used up to 1 month after opening. It should be discarded if the fluid changes colour or after 30 days.
- Check the expiry date on the vial and ensure the vial contains tuberculin PPD-S (5 TU/0.1 mL) or PPD RT 23 (2 TU/0.1 mL).
- Use a 1 mL graduated syringe or tuberculin syringe that can dispense 0.1 mL solution accurately using a short (8–13 mm) 27-gauge needle.
- Clean the top of the vial with a sterile swab.
- Draw 0.1 mL (5 TU) of tuberculin, or as per the manufacturer’s instructions, and expel air and excess drops.
- Tuberculin should be injected within 20 minutes of loading to the syringe.
- Inject tuberculin:
- After gentle cleaning of the site with an alcohol swab, stretch the selected area of the skin using the thumb and forefinger, insert the needle slowly with the bevel pointing upwards at an angleof 5–15 degrees, and advance the needle through the epidermis approximately 3 mm so the entire bevel is covered and visible just under the skin. Release the stretched skin and slowly inject tuberculin and check for leakage. If there is no leakage, continue to inject slowly until the complete 0.1 mL solution has been administered, and then remove the needle quickly.
- If a drop of blood appears, gently blot the injection site with alcohol-based disinfectant without squeezing out tuberculin.
- Check injection site:
- When the correct injection technique is used, a pale wheal measuring 6–10 mm in diameter will result. If the wheal is less than 6 mm in diameter, the test should be repeated at a site at least 5 cm away from the original site.
Reading
- The test should be read 48–72 hours after the injection (not before 48 hours or after 72 hours).
- Reading should be performed in good light, with the forearm slightly flexed at the elbow. The reader should gauge the presence of induration (palpable, raised, hardened area or swelling), starting with inspection and then palpation with light, gentle motion. Sweep fingertips over the surface of forearm in all four directions to locate margins or edges of induration. Using the fingertip as a guide, lightly mark the widest edges of the induration across the forearm with a fine line or dot. If the margins of the induration are irregular, mark and measure the widest diameter.
- The diameter of induration is measured across the forearm, from the thumb side of the arm to the little finger side. Using a plastic scale or ruler, place the zero ruler line inside the edge of marked fine line or dot and measure the ruler line inside the right dot or the alternate edge of the fine line. If the measurement falls between two divisions on the millimetre scale, record the lower division.
- Do not measure the diameter of the redness, swelling or bruising. Measure the induration. Alternatively, use the ballpoint pen method for reading. A ballpoint pen line may be drawn on the transverse axis of the forearm, starting 1–2 cm away from the visible skin test reaction and moving slowly towards its centre, exerting moderate pressure against the skin. The point where resistance to pen displacement occurs determines the outer limit of the induration. Mark lightly with a fine line or dot at the widest edges of the induration across the forearm and use a ruler to measure the diameter as above.
Recording
- Note the location of TST administration (right or left forearm).
- If there is no induration, record as “zero”. Otherwise, record the exact size of the induration in millimetres. Do not record as positive or negative.
- Record adverse events (if any) at the test site, such as formation of vesicles, bullae, lymphangitis, ulceration or necrosis.
Interpretation
TST does not measure immunity to TB but measures the degree of hypersensitivity to tuberculin. A skin test result is interpreted considering the person’s risk of being infected with TB and progression to disease when infected as well as size of the induration in millimetres. There is no correlation between the size of induration and likelihood of current TB disease (poor positive predictive value) or future risk of developing TB disease. There is no correlation between the size of TST reactions post-BCG vaccination and protection against TB disease. Overall, results of TST must be interpreted carefully considering individual clinical risk factors before determining the size of the induration that is positive (e.g. 5 mm, 10 mm or 15 mm). Details on interpretation are provided in Chapter 3.
Formation of vesicles, bullae, lymphangitis, ulceration and necrosis at the test site should be noted as they may indicate a high degree of tuberculin sensitivity and hence the presence of TB infection. A negative test may indicate lack of infection with M. tuberculosis or that the person has acquired infection recently and not enough time has elapsed for the body to react to the skin test. From the time of infection to the development of cell-mediated immunity, there is a window period of up to 12 weeks when TST would be negative. Most children with a negative result may not be infected with M. tuberculosis. Immunologically compromised individuals, especially people living with HIV and low CD4 T-cell counts or severe malnutrition, frequently show negative results from the PPD test. The absence of cell-mediated immunity to tuberculin may be due to lack of previous sensitization or due to anergy because of immune suppression.
Induration of diameter ≥5mm is considered positive in:
- children living with HIV;
- severely malnourished children (with clinical evidence of marasmus or kwashiorkor).
Induration of diameter ≥10 mm is considered positive in:
- all other children (whether or not they have received BCG vaccination).
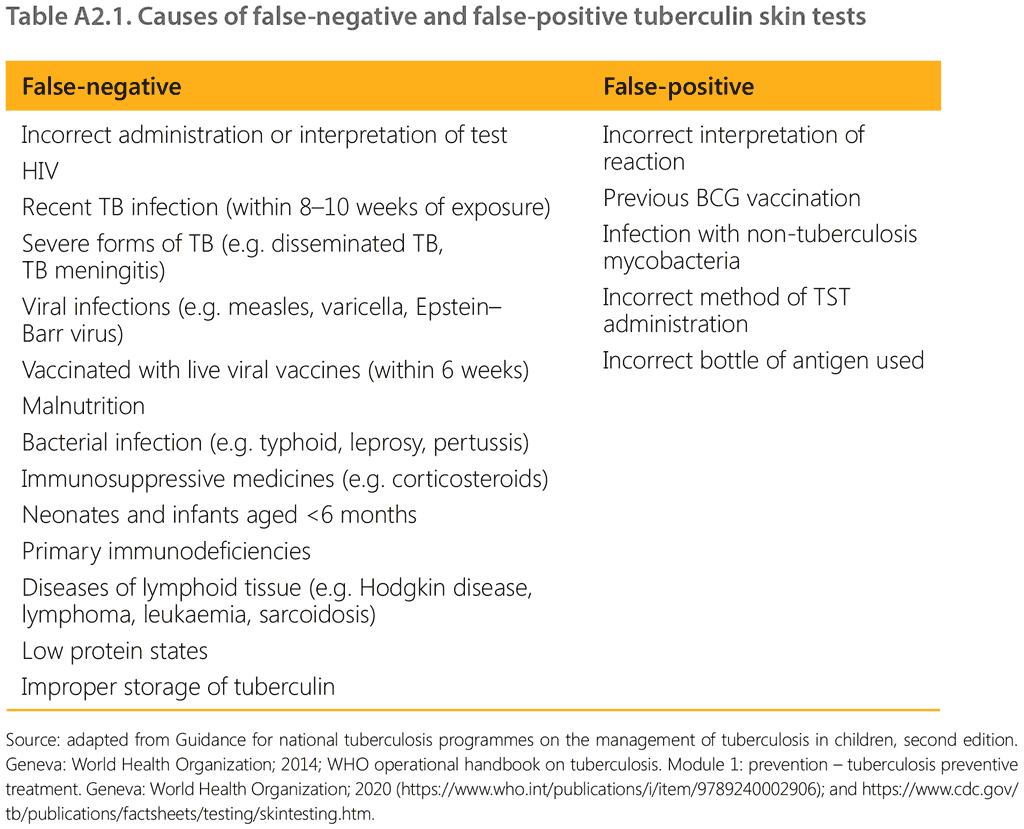
Causes of false-negative and false-positive TST are listed in Table A2.1.
 Feedback
Feedback