Book traversal links for 6. Safety and management of adverse drug reactions in TB preventive treatment

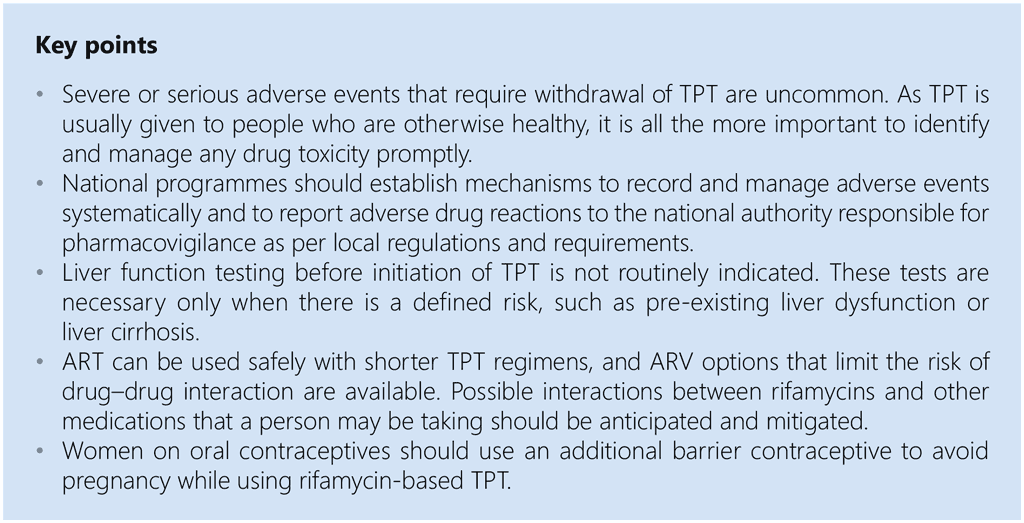
WHO has long recommended use of TPT for populations at risk of TB, particularly those with HIV and child household contacts of TB patients. Programmatic scaling up of TPT has nevertheless been limited in most high TB and HIV burden countries due to competing priorities. Concern about the efficacy and safety of TPT and interactions with other medicines, particularly ARVs, may also be barriers. Frequently raised questions include whether TPT will increase TB drug resistance in a community (see also section 5.6) and the durability of TPT (see also section 5.4) to protect against disease or mortality. This section presents the available evidence on some of these important issues to facilitate TPT uptake and programmatic scaling up.
 Feedback
Feedback