Book traversal links for 7.1.7.4. TPT in children and adolescents living with HIV on antiretroviral therapy
A key challenge with rifamycin-based TPT regimens in people living with HIV is drug–drug interactions. Rifampicin and rifapentine can be co-administered with EFV or DTG without dose adjustment. In people on RAL and rifamycins, however, a higher dosage of RAL (800 mg twice a day instead of 400 mg twice a day) should be used. This dose adjustment applies only to adolescents, as pharmacokinetic studies on the use of 3HP, 1HP and 3HR in children on various new ART regimens are ongoing. Rifampicin or rifapentine TPT regimens should not be co-administered with protease inhibitors or nevirapine. Further details on drug–drug interactions between TPT and ART medicines can be found in Chapter 6 of the WHO operational handbook on tuberculosis. Module 1: prevention – tuberculosis preventive treatment (15).
Table 7.3 summarizes the TPT options for people on ART.
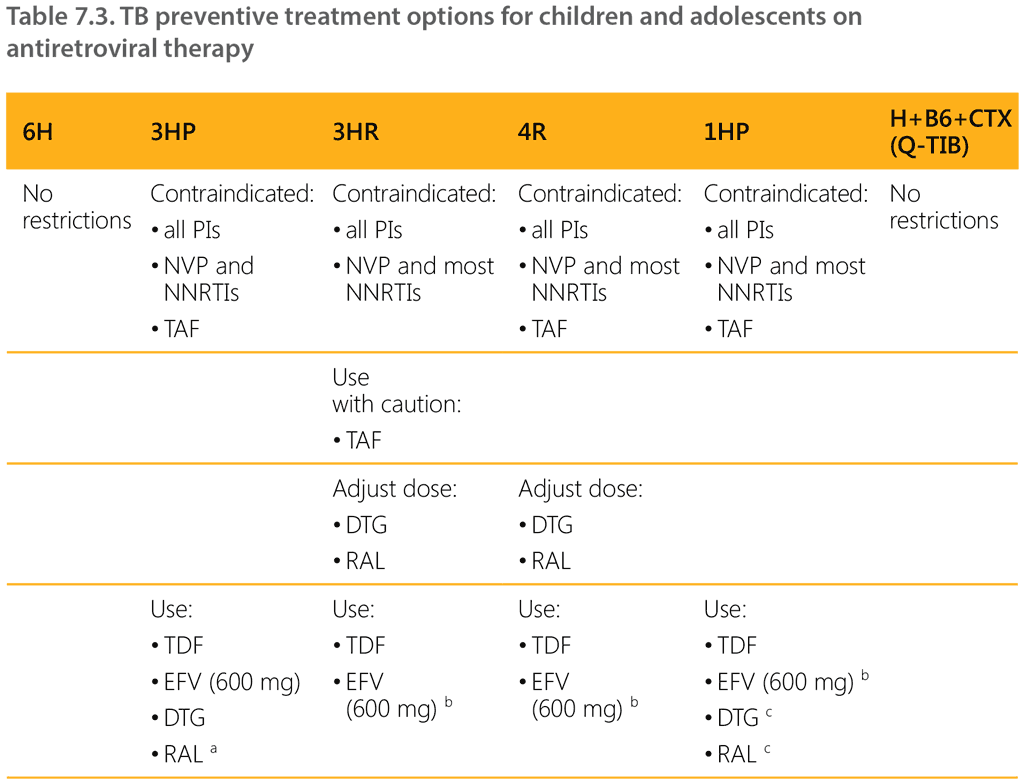
a Drug interaction has been studied in adults but not children; applies only to adolescents and adults taking DTG or RAL only.
b EFV 600 mg applies to adolescents and adults; EFV is not recommended in children aged under 3 years.
c For adolescents on 1HP who are taking DTG or RAL, dosing of DTG and RAL needs to be adjusted as per Table 7.2.
Abbreviations: B6: pyridoxine; CTX: co-trimoxazole; DTG: dolutegravir; EFV: efavirenz; H: isoniazid; NNRTIs: non-nucleoside reversetranscriptase inhibitors; NVP: nevirapine; PIs: protease inhibitors; RAL: raltegravir; TAF: tenofovir alafenamide; TDF: tenofovir disoproxil fumarate.
 Feedback
Feedback