Book traversal links for 3.3.6. Options for TB preventive treatment regimens: drug-resistant TB
Household contacts of people with MDR-TB or isoniazid monoresistance are at higher risk of TB infection than contacts exposed to people with drug-susceptible TB. The risk of progression to TB disease does not differ among contacts in either group (67). Studies have reported approximately 90% reduction in MDR-TB incidence with TPT after known exposure (68). WHO recommends using TPT for contacts exposed to people with MDR-TB following consideration of the intensity of exposure, confirming the source patient and their drug resistance pattern (i.e. MDR-TB confirmed bacteriologically and susceptibility to a fluoroquinolone established), and confirming TB infection using IGRA or TST where possible.
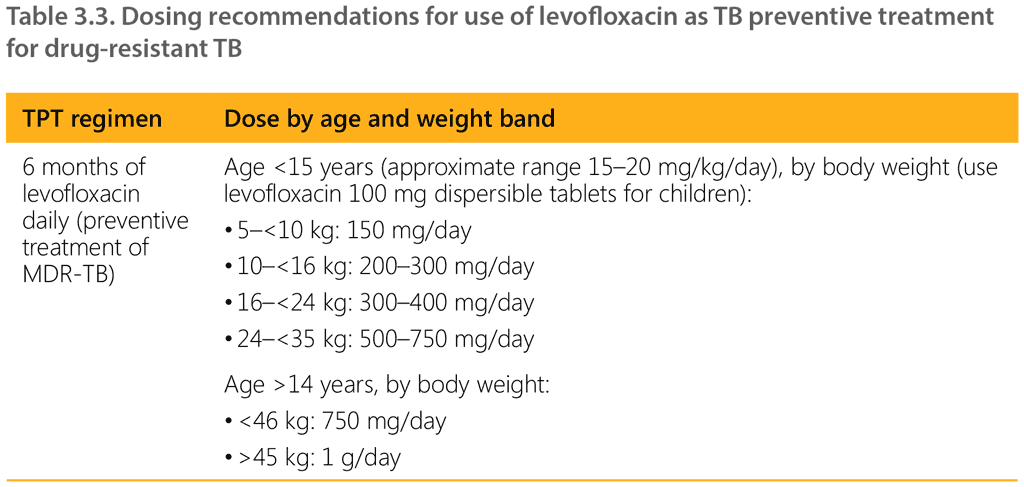
WHO suggests the use of levofloxacin for 6 months (paediatric formulation for child contacts) along with other TB medicines such as ethambutol (or ethionamide if tolerated). Regardless of whether treatment is given or not, clinical follow-up should be carried out for 2 years. Any emergent signs and symptoms suggestive of TB should be actively investigated and a treatment regimen started as needed. Table 3.3 provides dosing recommendations for the use of levofloxacin as TPT for contacts of DR-TB.
Contacts of people with rifampicin-resistant TB (RR-TB) may be treated similarly to contacts of people with MDR-TB. If isoniazid susceptibility is confirmed in the index patient, contacts may be given 6H or 9H. Among contacts of people with known isoniazid-resistant rifampicin-susceptible TB, little evidence on the choice of TPT regimens exists. 4R may be an option for TPT in these situations.
 Feedback
Feedback