Book traversal links for 2.3 Evidence to recommendations: considerations
In 2022, new evidence from programmatic implementation in South Africa was made available to WHO where the regimen was modified to include 2 months of linezolid (600 mg) instead of 4 months of ethionamide.
Based on an assessment of the certainty of the evidence, carried out using predefined criteria and documented in the GRADEpro software, the certainty of the evidence was rated as very low for both comparisons.
Table 2.1 lists the comparisons and decisions on each of the sub-PICO questions that were assessed by the GDG to conclude with the summary recommendation (Recommendation 2.1). The main assessment that defined the overall decision was based on sub-PICO 1.1. The background for this decision was provided by the previous review and recommendation for the use of the 9-month regimen with ethionamide agreed during the GDG meeting in November 2019 and reflected in the recommendations published in the 2020 DR-TB treatment guidelines update (41).
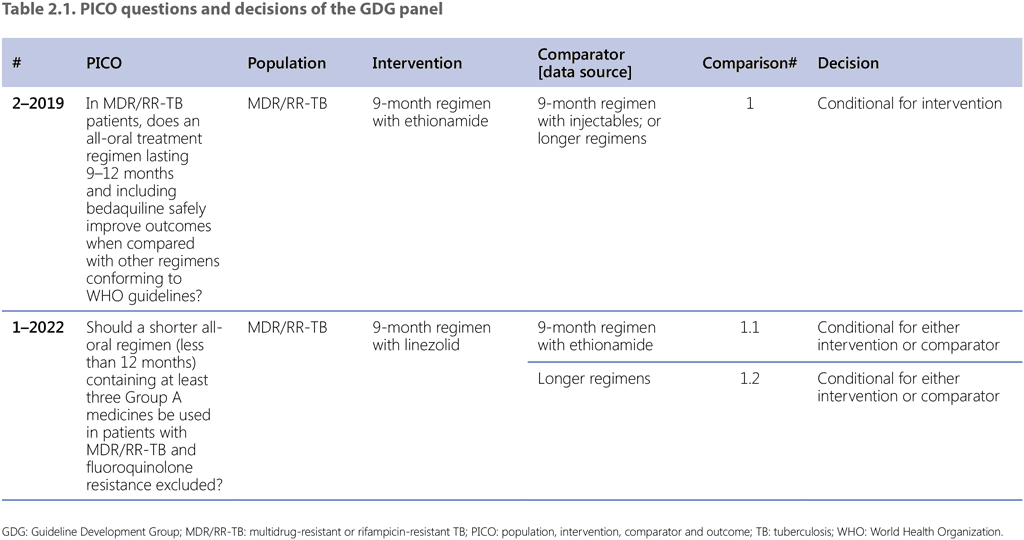
Sub-PICO 1.1
The GDG acknowledged that, during the analysis, the intervention and comparator groups were made as comparable as possible. However, the GDG considered possible unmeasured confounding due to a lack of systematic collection of information on comorbidities and radiological findings through the EDRWeb system, as well as methodological challenges (e.g. a potential selection bias). Apart from the selection criteria listed, the risk of major selection bias was considered to be low, given that this intervention represented a complete and comprehensive switch in the countrywide programmatic approach.
Regarding generalizability, the GDG deliberated whether the genetic diversity of M. tuberculosis strains in South Africa was comparable to strains present in other settings; the group concluded that strains found in other settings were adequately represented in the country. The group also considered potential interactions in relation to HIV status and the effect of ART, but this was not considered a major factor given that treatment outcomes were similar in People with HIV and HIV-negative people. The GDG agreed that results of the STREAM Stage 2 trial – a large-scale, multicountry Phase 3 trial examining a shorter all-oral bedaquiline-containing regimen – will provide additional important insight into the efficacy and safety of this regimen, and may increase the certainty of the evidence.
A clear limitation emphasized by the GDG was the lack of data on adverse events in the EDRWeb. No direct comparative evidence was available on adverse events because the data on such events were not systematically collected for the 9-month regimen with ethionamide. The rate of Grade 3–5 adverse events was 5% for the 9-month regimen with linezolid. The panel nevertheless considered the potential adverse events of both ethionamide and linezolid in balancing the benefits and harms (Table 2.2).
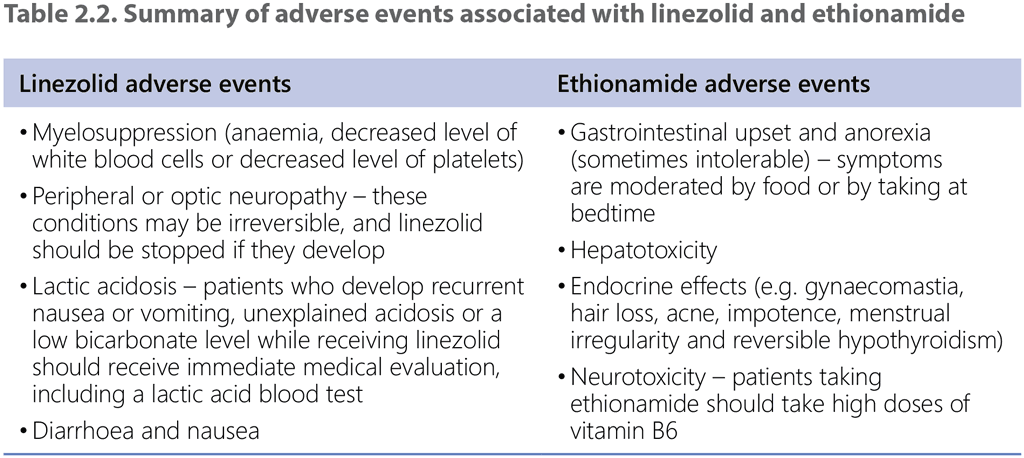
The panel also considered the duration and pill burden with the intervention and comparator regimens. Both regimens have the same duration, so neither offers an advantage of shorter treatment, although the duration of the linezolid regimen is shorter than that of ethionamide. The pill burden is likely to be slightly lower with the intervention because linezolid is prescribed for 2 months in the 9-month regimen with linezolid and ethionamide for 4 months in the 9-month regimen with ethionamide.
Considering this evidence, the panel judged that the 9-month regimen with linezolid may have small desirable effects and noted the very low certainty of the evidence. Certainty of the evidence was rated “very low” for all outcomes on account of potential misclassification bias and confounding bias (downgraded 1 level), and serious indirectness (downgraded 1 level). The overall certainty is generally based on the lowest certainty for the agreed critical outcomes; thus, it was judged to be very low. The panel noted that the evidence on both the intervention and on the comparator regimen was obtained from programmatic data from South Africa such that, overall, the population and health care context were comparable. However, the panel stressed that important differences exist between the two cohorts or datasets that were compared, making it difficult to draw conclusions with full confidence.
The panel judged that there was probably no important uncertainty or variability in how much people value the main outcomes. The panel used available data on cost of component medicines combined with professional judgement to estimate the cost of the 9-month regimen with linezolid compared with the 9-month regimen with ethionamide among patients with MDR/RR-TB, susceptible to fluoroquinolones. The panel suggested that the cost would be expected to be very similar; that is, for there to be negligible costs or savings. The panel also noted that no data were available on the cost of managing potential long-term consequences of neurotoxicity that can be caused by the use of linezolid, and that the risk is greater if linezolid is used for longer periods. The panel has also noted that health care and patient costs are likely to be similar for regimens when used in a similar group of patients and for the same duration.
The GDG attempted to discuss cost–effectiveness of the two regimens; however, no evidence was available, the two regimens are identical in duration and they only differ in one component drug, which would not change the overall cost of the regimen in any significant way. The similarity of the two regimens also prevented a substantial discussion on the equity. The panel considered patients and health care providers as key stakeholders. The panel considered the following aspects as critical with regard to acceptability: regimen duration, drug-safety monitoring needs (relating both to the necessary travel, loss of income and general disruption of the life of patients, and to workload for the health care system) and DST needs. The panel judged that there were probably no differences in acceptability between the 9-month regimen with linezolid and the 9-month regimen with ethionamide, given the overall similarity of the regimens, and that the 9-month regimen with linezolid would probably be acceptable. The panel considered the following aspects to affect feasibility (i.e. to be potential barriers to implementation): requirements for drug-safety monitoring and for DST. The 9-month regimen with linezolid would require monitoring of toxicity (e.g. anaemia) and DST.
The panel judged that the balance of desirable and undesirable consequences favours neither the 9-month regimen with linezolid nor the 9-month regimen with ethionamide in this population. Specifically, the panel felt that there is a fine balance between the two options in terms of benefits and harms that is uncertain given the overall very low certainty in the evidence (due to potential misclassification bias, confounding bias and serious indirectness). The panel judged that for most other evidence-to-decision criteria (e.g. resources, acceptability and feasibility) there was unlikely to be a large difference between the 9-month regimen with linezolid and the 9-month regimen with ethionamide because the only difference between the two regimens is the replacement of ethionamide with linezolid. Overall, the panel judged that either regimen could be used and that the flexibility of using either linezolid or ethionamide was helpful to optimize patient care. These considerations also guided the agreement of the panel on the strength of the recommendation being conditional.
Sub-PICO 1.2
The GDG acknowledged that, during the analysis, the intervention and comparator groups were made as comparable as possible. The panel noted that the evidence on the 9-month regimen was obtained from programmatic data from South Africa, whereas the evidence on the longer regimen represented only subsets of patients from the countries and researchers that submitted data. The panel also noted substantial inconsistency between cohorts in the comparator group (on the longer regimens). Overall, there was concern that the selective nature of the data on the longer regimens may have biased the comparison in favour of the longer regimen. As a result, there were serious concerns about the comparability of the data, making it difficult to draw conclusions with confidence. The panel also considered the duration and overall pill burden with the intervention and comparator regimens, which are both lower in the 9-month regimen and thus represent a benefit of the intervention.
Considering this evidence and the totality of observed effects of the 9-month regimen with linezolid on the outcomes, the panel judged that the 9-month regimen with linezolid may have moderate desirable effects and that it may also have moderate undesirable effects.
Certainty in the estimates was rated “very low” for all outcomes owing to very serious risk of bias (potential misclassification bias and confounding bias), inconsistency (inconsistency in the effect estimates among 14 comparator cohorts) and indirectness (with data for the intervention regimen being from a single country). The overall certainty is generally based on the lowest certainty for the agreed critical outcomes and thus was judged to be very low.
The panel noted that the costs for people with MDR/RR-TB receiving the 9-month regimen with linezolid are expected to be lower than those for longer regimens (18 months or longer) because costs for drugs, care and monitoring are expected to be lower.
The panel considered the ability to decentralize treatment (to enable access for remote, underserviced settings and disadvantaged populations) as affecting equity. Despite not being able to identify relevant research evidence, the panel used their collective experience to judge that there would probably be advantages associated with the use of the 9-month regimen owing to its reduced complexity and shorter duration. The panel judged that use of the 9-month regimen with linezolid would probably increase equity.
The panel considered patients and health care providers as key stakeholders and the following aspects as critical with regard to acceptability: regimen duration and drug safety, monitoring needs (relating both to the necessary travel, loss of income and general disruption of the life of patients, and to workload for the health care system) and needs for DST. The panel judged that the 9-month regimen with linezolid would probably be acceptable to key stakeholders.
The balance of desirable and undesirable consequences was judged to not favour either the use of the 9-month regimen or the longer, 18-month regimens in this population. Specifically, the panel felt that there is a fine balance between the two options in terms of benefits and harms that is uncertain given the overall very low certainty in the evidence. The panel judged that although the balance of effects did not favour either the intervention or the comparator, several other evidence-to-decision table criteria (e.g. resources, acceptability, equity and feasibility) favoured the 9-month regimen.
Overall, the panel judged that either regimen could be used in the eligible patient group presented in the analysis; they noted the more limited eligibility for the 9-month regimen and acknowledged that the applicability of the longer, individualized regimens is more flexible and significantly broader, including many patient groups that are not eligible for the shorter regimen. These considerations have also guided the agreement of the panel on the conditionality of this recommendation.
 Feedback
Feedback