Book traversal links for 1.4.2. Safety
A systematic review of studies reporting the outcomes of interest, including local reactions – that is, injection site reactions (ISR) and systemic adverse events from TBSTs – was undertaken. The following databases were searched for studies from inception until 30 July 2021: Medline, Embase, e-library, the Chinese Biomedical Literature Database and the China National Knowledge Infrastructure Database. The test manufacturers were contacted for individual studies, and studies were identified through a public call for data by WHO. Longitudinal and case–control studies reporting adverse events of the index tests alone or compared with recognized comparator tests (e.g. QFT, T-Spot and the TST) in humans were included with no language restrictions. Screening of titles and abstracts as well as full-text articles and the assessment of quality were performed by two investigators in duplicate. A meta-analysis was conducted using a random-effects model, and studies that were considered to be clinically homogenous were pooled.
Overall, seven studies for Cy-Tb, five for C-TST and 11 for Diaskintest were identified.
Characteristics of studies were as follows:
- Cy-Tb: clinical trials – three studies in South Africa and four in Europe. Most participants were adults; in studies in South Africa, 20–40% of participants were People with HIV. Five of seven studies included random allocation of Cy-Tb versus the TST into two arms and thus allowed comparison of ISR. All five studies were included in the pooled evidence assessment on any ISR. Only one study provided comparable data on systemic reactions. This study was also included in the pooled evidence assessment on systemic reactions.
- C-TST: all five studies were conducted in China and included only HIV-negative adults. All of them included non-random allocation of C-TST versus the TST into two arms; thus, no study evaluating C-TST was included in the pooled evidence assessment on any ISR. Also, no studies including any comparable data on systemic reactions were available.
- Diaskintest: cross-sectional studies using routinely collected data mostly in the Russian Federation, and one in Ukraine, including various populations (adults, children and adolescents – healthy, contacts of TB patients and with TB). Two studies on Diaskintest provided comparable data on ISR; however, one of them provided no information about the number of participants who experienced any ISR; thus, only one study on Diaskintest was included in the meta-analysis.
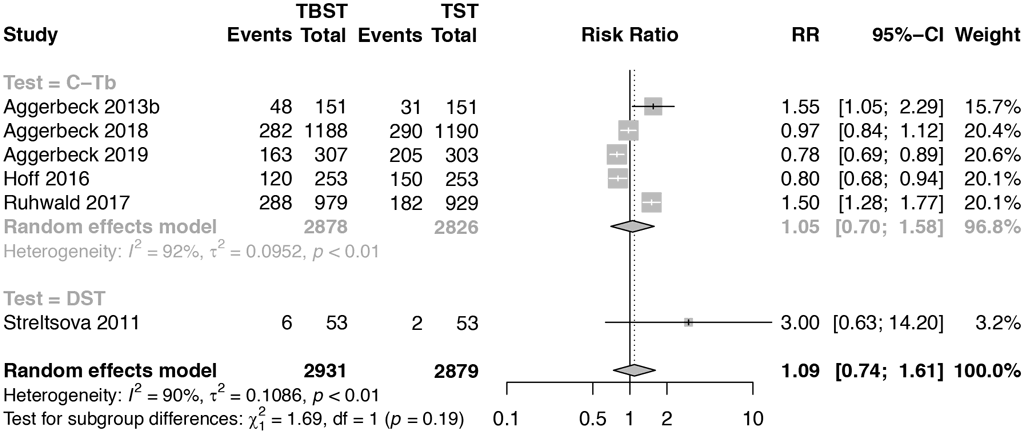
Fig. 10. Any injection site reactions
CI: confidence interval; DST: drug susceptibility testing; HIV: human immunodeficiency virus; People with HIV: people living with HIV; RR: risk ratio; TB: tuberculosis; TBSTs: Mycobacterium tuberculosis antigen-based skin tests; TST: tuberculin skin test.
Proportion of People with HIV: Aggerbeck 2018 (7) (25%), Aggerbeck 2019 (8) (20%); Hoff 2016 (10) (39.5%). Other studies included HIV-negative individuals. Aggerbeck 2018 (7) included children aged under 5 years (20%) and aged 5–17 years (31%); Ruhwald 2017 (9) included children aged under 5 years (3.5%) and aged 5–17 years (8.8%). Other studies included adults. Hoff 2016 (10), Aggerbeck 2019 (8) and Streltsova 2011 (11) included people with TB only.
The pooled risk of any ISR due to Cy-Tb (n=2878, 5 studies) and Diaskintest (n=53, 1 study) presented in Fig. 10 was not significantly different from the TST (risk ratio [RR] 1.09; 95% CI: 0.74–1.61). The risk of any systemic reaction was only analysable in one study (Cy-Tb) that allowed such comparison, and was not significantly different from the TST (RR 0.84; 95% CI: 0.60–1.10). The Diaskintest study was considered to have high risk of bias, while the overall certainty of evidence from the randomized controlled trials for any ISR was judged as high. For any systemic reactions, overall certainty of evidence was judged to be moderate because of the small sample size and wide CI.
Following the request from GDG members for the post-marketing surveillance data for Diaskintest, the following data were reported by the manufacturer: in 2019–2021, over a 55.7 mln Diaskintest tests were done, with 27 serious adverse effects and 30 non-serious adverse effects. Based on the totality of data, the GDG rated the certainty of evidence as high.
Based on the data presented at the GDG meeting, it was concluded that the safety profile of novel TBSTs is similar to that of the TST, and is associated with mostly mild ISR such as itching and pain. From the reviewed studies, there appears to be no safety signal that might affect the choice between specific TBSTs and the TST. However, the group also noted that this was not a full safety review covering product safety, animal or preclinical studies. Regulatory assessment for safety is needed before any of the TBST products are implemented.
 Feedback
Feedback