Book traversal links for 4.5.1 Implementing a new diagnostic algorithm
Modifications to diagnostic algorithms must be put in place only after a formal evaluation, review and approval by officials within the MoH, NTP and NTRL. Often, nationally appointed thematic working groups are used to evaluate new technologies and develop implementation plans, which typically include revising current algorithms. These groups comprise local ministry officials, implementing partners, civil society and professionals (laboratory and medical), who will decide the optimal use and placement of the new technology within the current network structure. The following points should be considered when designing or reviewing algorithms for testing at different levels of the laboratory network:
- the specific diagnostic tests in use or being considered for use;
- whether the tests are recommended by WHO and, if so, for what purposes;
- the ability to collect the specimens required for the test;
- what additional testing is recommended to follow up the results of the new tests;
- the current and planned capacity of the country’s laboratories, laboratory infrastructure and availability of competent personnel to conduct the tests;
- the adequacy of systems for specimen collection and transport;
- the capacity of clinical services to offer diagnosis and treatment;
- the drugs used for the treatment of TB and DR-TB in the country; and
- the characteristics of the population (i.e. the groups at risk) being served, which should be derived from population-based studies (if available), including the proportion of people with DR-TB, PLHIV and people with extrapulmonary TB, and the proportion of TB among children.
Algorithms should be designed to use existing laboratory services and networks, so that specimens can be referred to the appropriate level for tests that are not available at peripheral-level laboratories. Such referrals are particularly important when evaluating individuals for DR-TB or HIV-associated TB, evaluating children for TB or evaluating individuals for extrapulmonary disease.
Fig. 4.8. Scenario 1: High TB/HIV setting
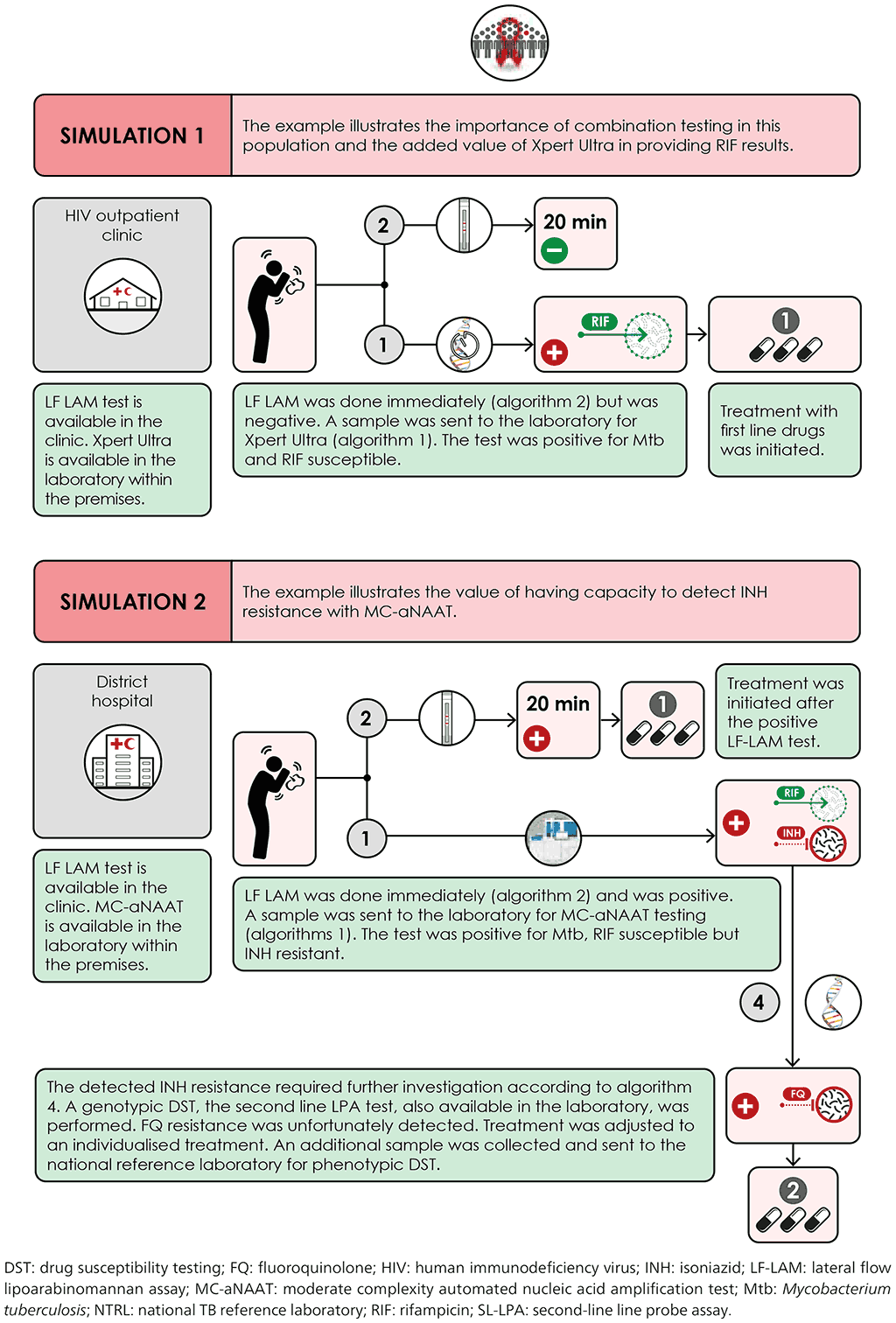
Fig. 4.9. Scenario 2: High Hr-TB setting
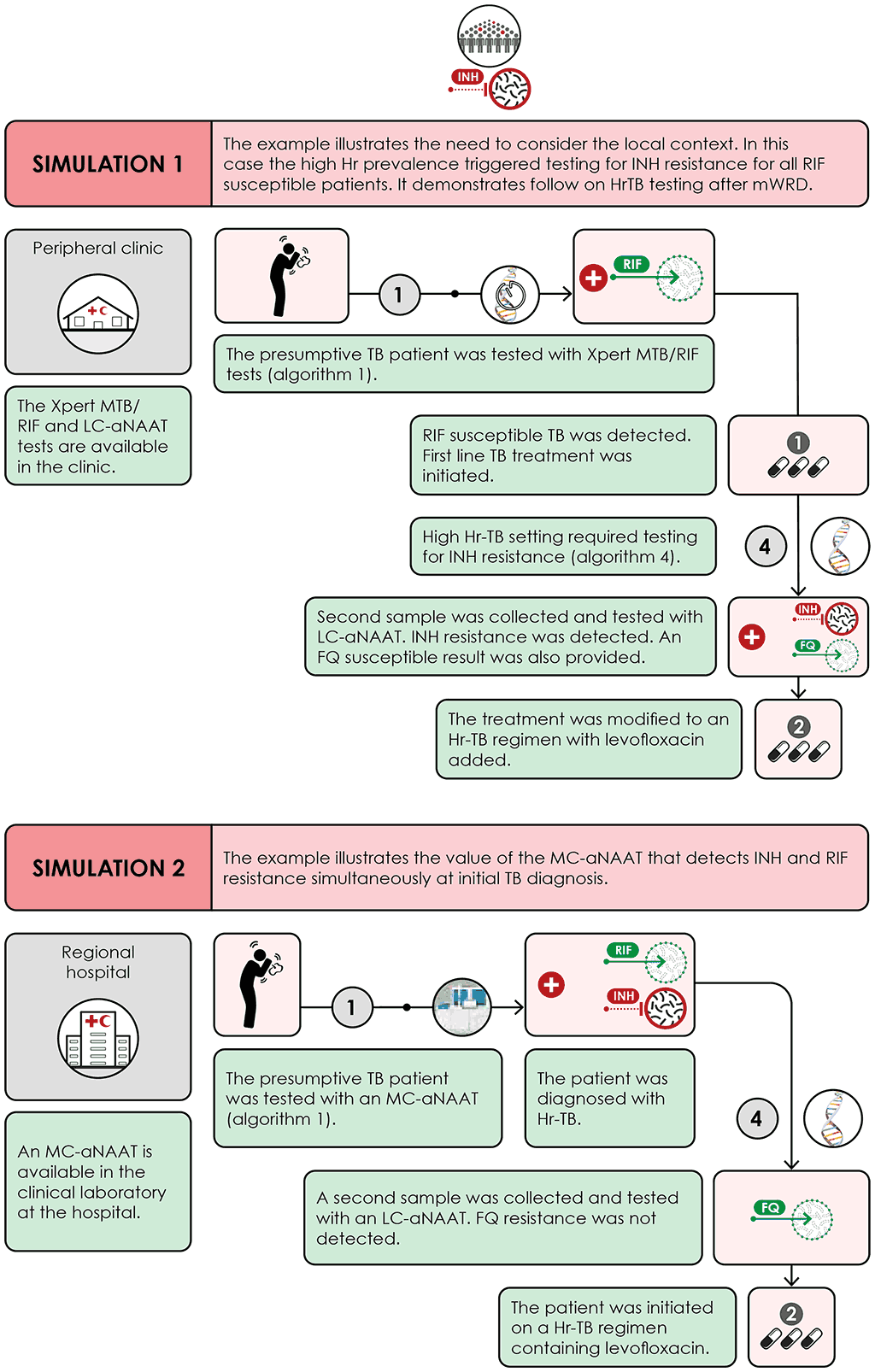
FQ: fluoroquinolone; Hr-TB: isoniazid-resistant, rifampicin-susceptible TB; INH: isoniazid; LC-aNAAT: low complexity automated nucleic acid amplification test; LFX: levofloxacin; MC-aNAAT: moderate complexity automated nucleic acid amplification test; mWRD: molecular World Health Organization–recommended rapid diagnostic test; RIF: rifampicin; TB: tuberculosis.
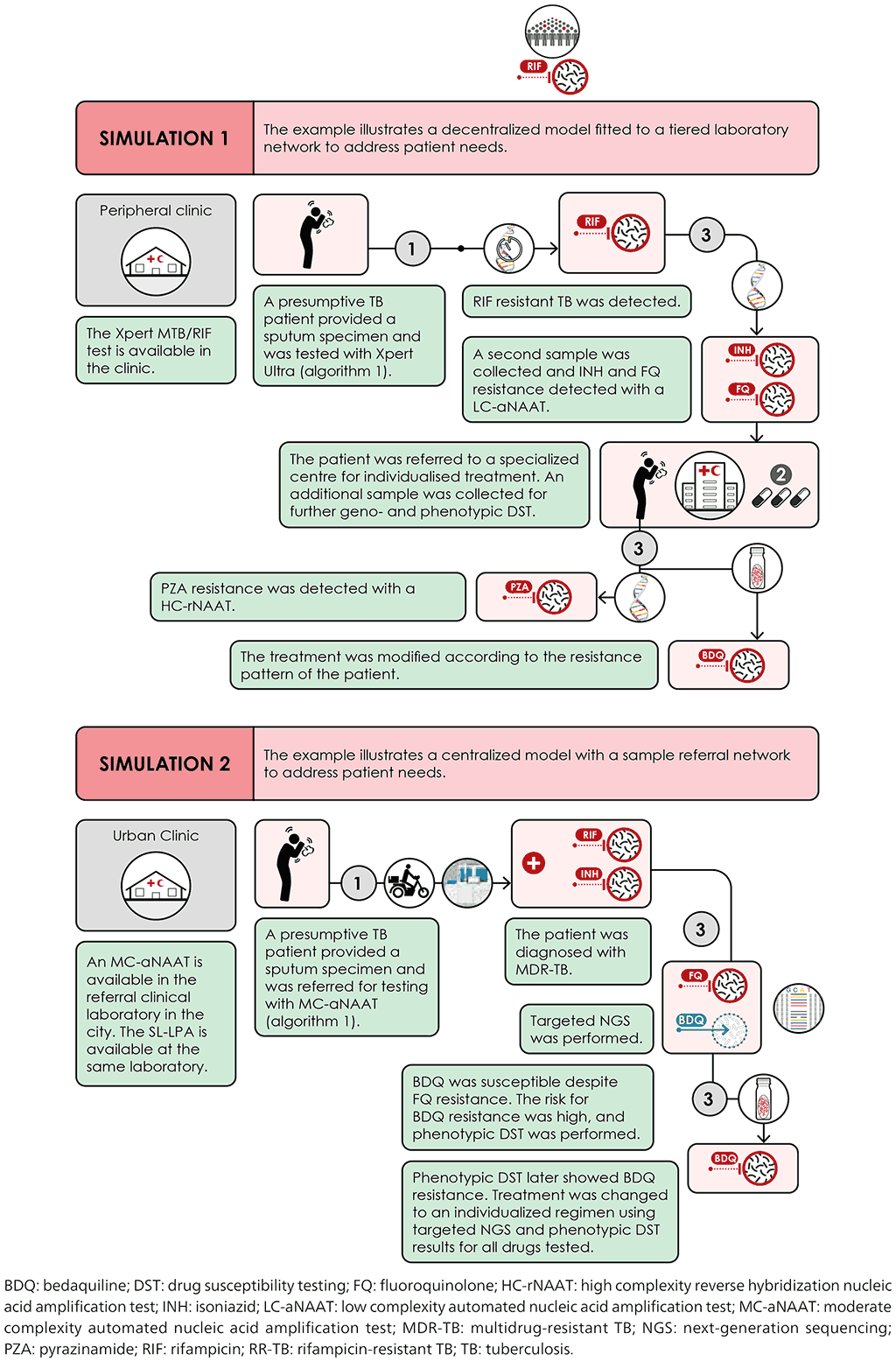
Fig. 4.10. Scenario 3: High MDR/RR-TB setting
 Feedback
Feedback