Breadcrumb
Key messages
Book navigation
-
Consolidated Guidelines
-
Module 1: Prevention
-
Module 1: TB Preventive Treatment
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
-
Introduction
▶
▶
▶
▶
-
1. Recommendations
- 1.1. Identifying populations for TB preventive treatment
- 1.2 TB screening and ruling out TB disease
- 1.3 Testing for TBI
- 1.4 TB preventive treatment options
▶
▶
▶
▶
- 2. Monitoring and evaluation
- 3. Research gaps
- 4. References
- Annex 1. Recommendations in the WHO consolidated guidelines on tuberculosis: tuberculosis preventive treatment, second edition (2024) and in the previous edition (2020)
- Annex 2. Methods and expert panels
- Annex 3. GRADE summary of evidence tables
- Annex 4. GRADE evidence-to-decision tables
-
Annex 5. Summary of unpublished studies (PICO 10)
- A5.1 Summary of TB CHAMP and V-QUIN clinical trials
- A5.2 Use of fluoroquinolones for TB preventive treatment in contacts of persons with MDR-/RR-TB: A systematic review
- A5.3 Assessing fluoroquinolone (levofloxacin) acceptability among contacts of MDR-TB patients: a qualitative study10
- A5.4 A survey to explore the programmatic feasibility of levofloxacin (Lfx) TPT for MDR-TB contacts11
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 1: TB infection prevention and control
- Abbreviations
- Glossary
- Acknowledgements
- Declarations of interest
- How to use these guidelines
-
Executive summary
▶
▶
▶
▶
-
1. Introduction
▶
▶
▶
▶
-
2. Recommendations
▶
▶
▶
▶
- 3. Core components of IPC programmes
-
4. Methods
- 4.1. Preparation for evidence assessment
- 4.2. Evidence retrieval, quality assessment and grading of the evidence
- 4.3. Formulation of the recommendations
- 4.4. Guideline Development Group decision-making
- 4.5. Guideline preparation, peer-review and content presentation
▶
▶
▶
▶
- 5. Research priorities
- 6. Publication, dissemination and implementation
- References
-
Annexes
- Annex 1 – List of participants to the Guideline Development Group meeting
- Annex 2 – Summary of declarations of interest and management
- Annex 3 – Risk of acquiring tuberculosis infection, progression to active disease and the effect of treatment on infectiousness
▶
▶
▶
▶
-
Online annexes
- Annex 4 – GRADE evidence summary tables
- Annex 5 – GRADE evidence-to-decision tables
- Annex 6 – Results of the systematic reviews on the development of these guidelines
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 1: TB Preventive Treatment
-
Module 2: Screening
-
Module 2: Systematic screening for tuberculosis disease
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
-
1. Introduction
- 1.1 Background
- 1.2 Definition and objectives of systematic screening for TB disease
- 1.3 Scope of the 2021 update
- 1.4 Rationale for the guideline update
- 1.5 Objectives of the guideline update
- 1.6 Target audience
▶
▶
▶
▶
-
2. Recommendations for systematic screening for TB disease in targeted populations
-
2.1 Systematic screening for TB disease among the general population
▶
▶
▶
▶
-
2.2 Systematic screening for TB disease among people with structural risk factors for TB
▶
▶
▶
▶
-
2.3 Systematic screening for TB disease among people living with HIV
▶
▶
▶
▶
-
2.4 Systematic screening for TB disease among household and other close contacts of individuals with TB disease
- 2.4.1 Summary of evidence and rationale
- 2.4.2 Implementation considerations
- 2.4.3 Subgroup considerations
▶
▶
▶
▶
-
2.5 Systematic screening for TB disease in prisons and other penitentiary institutions
▶
▶
▶
▶
-
2.6 Systematic screening for TB disease among miners and others exposed to silica dust
▶
▶
▶
▶
-
2.7 Systematic screening for TB disease among people attending health care services who have clinical risk factors for TB
▶
▶
▶
▶
▶
▶
▶
▶
-
2.1 Systematic screening for TB disease among the general population
-
3. Recommendations for tools for systematic screening for TB disease
-
3.1 Tools for screening for TB disease among the general population and high-risk groups
▶
▶
▶
▶
-
3.2 Use of computer-aided detection software for automated reading of digital chest radiographs
▶
▶
▶
▶
-
3.3 Tools for screening for TB disease among people living with HIV
-
3.3.1 Summary of the evidence and rationale
- 3.3.1.1 WHO-recommended four-symptom screen
- 3.3.1.2 C-reactive protein
- 3.3.1.3 Chest radiography
- 3.3.1.4 Molecular WHO-recommended rapid diagnostic tests for medical inpatients living with HIV in settings with a high TB burden
- 3.3.1.5 Molecular WHO-recommended rapid diagnostic tests for all other people living with HIV
▶
▶
▶
▶
- 3.3.2 Implementation considerations for all tools for screening people living with HIV
▶
▶
▶
▶
-
3.3.1 Summary of the evidence and rationale
-
3.4 Tools for systematic screening for TB disease among children and adolescents
-
3.4.1 Summary of the evidence and rationale
- 3.4.1.1 Close contacts younger than 15 years
- 3.4.1.2 Children younger than 10 years who are living with HIV
▶
▶
▶
▶
- 3.4.2 Considerations for screening children and adolescents
▶
▶
▶
▶
-
3.4.1 Summary of the evidence and rationale
▶
▶
▶
▶
-
3.1 Tools for screening for TB disease among the general population and high-risk groups
-
4. Monitoring and evaluation
- 4.1 Indicators
- 4.2 Routines for recording and reporting
- 4.3 Programmatic evaluations
- 4.4 Initial calibration for computer-aided detection technologies
▶
▶
▶
▶
-
5. Research gaps
-
5.1 Screening for TB in targeted populations
- 5.1.1 The general population and high-risk groups
- 5.1.2 People living with HIV
- 5.1.3 Children and adolescents
▶
▶
▶
▶
-
5.2 Tools for screening for TB
▶
▶
▶
▶
- 5.3 Operational research
▶
▶
▶
▶
-
5.1 Screening for TB in targeted populations
- 6. References
- Supplementary Table
-
Web annexes
- Web Annex A. Methods and Expert Panels
- Web Annex B. GRADE Summary of Findings Tables
- Web Annex C. GRADE Evidence to Decision Tables
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 2: Systematic screening for tuberculosis disease
-
Module 3: Diagnosis
-
Module 3: Rapid diagnostics for tuberculosis detection
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
-
1. Introduction
▶
▶
▶
▶
-
2. Recommendations
-
2.1 Initial diagnostic tests for diagnosis of TB with drug-resistance detection
- Xpert MTB/RIF and Xpert MTB/RIF Ultra assays
- Truenat MTB, MTB Plus and MTB-RIF Dx assays
- Moderate complexity automated NAATs for detection of TB and resistance to rifampicin and isoniazid
▶
▶
▶
▶
-
2.2. Initial diagnostic tests for diagnosis of TB without drug-resistance detection
▶
▶
▶
▶
-
2.3 Follow-on diagnostic tests for detection of additional drug-resistance after TB confirmation
- Low complexity automated NAATs for detection of resistance to isoniazid and second-line anti-TB agents
- First-line LPAs
- Performance of SL-LPA on sputum specimens and culture isolates
- High complexity reverse hybridization-based NAATs for detection of pyrazinamide resistance
- Targeted next-generation sequencing
▶
▶
▶
▶
▶
▶
▶
▶
-
2.1 Initial diagnostic tests for diagnosis of TB with drug-resistance detection
- Research gaps
- References
-
Annexes
- Annex 1: Guideline development methods
- Annex 2: Conflict of interest assessment for Guideline Development Group and External Review Group members
- Annex 3: Guideline development group members
- Web Annex A. List of studies included in systematic review
- Web Annex B. GRADE profiles
- Web Annex C. Evidence to decision tables
- Web Annex D. Evidence synthesis and analysis
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 3: Tests for TB infection
- Acknowledgements
- Abbreviations and acronyms
- Executive summary
-
1. Use of Mycobacterium tuberculosis antigen-based skin tests for the diagnosis of TB infection
- 1.1. Background
- 1.2. Recommendation
- 1.3. Test descriptions
-
1.4. Evidence base
- 1.4.1. Diagnostic accuracy
- 1.4.2. Safety
- 1.4.3. Cost and cost–effectiveness analysis
- 1.4.4. User perspective
▶
▶
▶
▶
- 1.5. Implementation considerations
- 1.6. Monitoring and evaluation
- 1.7. Research priorities
▶
▶
▶
▶
-
2. Use of the TST and IGRAs for the diagnosis of TB infection
- 2.1. Background
- 2.2. Recommendation
- 2.3. Test descriptions
-
2.4. Evidence base
- 2.4.1. PICO question
- 2.4.2. Evidence on intervention effect
- 2.4.3. Cost–effectiveness
- 2.4.4. User perspective
▶
▶
▶
▶
- 2.5. Implementation considerations
- 2.6. Research priorities
▶
▶
▶
▶
-
3. Use of the TST and IGRAs for the diagnosis of TB disease
- 3.1. Background
- 3.2. Recommendation
- 3.3. Test descriptions
-
3.4. Evidence base
- 3.4.1. PICO questions
- 3.4.2. Hierarchy of reference standards
- 3.4.3. Studies search, selection and quality assessment
- 3.4.4. Data synthesis and meta-analysis
- 3.4.5. Use of IGRAs in the diagnosis of active TB
- 3.4.6. Operational aspects of the use of IGRAs
▶
▶
▶
▶
- 3.5. Research priorities
▶
▶
▶
▶
- References
- Annex 1. Summary of changes between the 2011–2020 guidance and the 2022 update
- Annex 2. GDG processes and decision-making
- Annex 3. Conflict of interest assessment for Guideline Development Group and External Review Group members
-
Web annexes
- Web Annex A. Accuracy of Mycobacterium tuberculosis antigen-based skin tests: a systematic review and meta-analysis
- Web Annex B. Safety of Mycobacterium tuberculosis antigen-based skin tests: a systematic review and meta-analysis
- Web Annex C. GRADE profiles of Mycobacterium tuberculosis antigen-based skin tests
- Web Annex D. Cost–effectiveness of Mycobacterium tuberculosis antigen-based skin tests: a systematic review
- Web Annex E. Modelling for economic evidence for the use of Mycobacterium tuberculosis antigen-based skin tests
- Web Annex F. Qualitative evidence for the use of Mycobacterium tuberculosis antigen-based skin tests
- Web Annex G. Mycobacterium tuberculosis antigen-based skin tests: evidence-to-decision table
- Web Annex H. Use of tuberculin skin test or interferon gamma release assays for identifying individuals at greatest risk of progression to active TB
- Web Annex I. Diagnostic accuracy of interferon gamma release assays for evaluation of patients with pulmonary TB
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 3: Rapid diagnostics for tuberculosis detection
-
Module 4: Treatment
-
Module 4: Drug-susceptible tuberculosis treatment
- Acknowledgements
- List of abbreviations
- Definitions
- Executive summary
- Introduction
- Objectives
-
Methods used to update the guidelines
- Scope of the guideline update
- Certainty of evidence and strength of recommendations
- Assessment of evidence and its grading
- External review
- Publication, dissemination, implementation, evaluation and expiry
▶
▶
▶
▶
-
Recommendations
- Treatment of drug-susceptible TB using 6-month regimen
- Treatment of drug-susceptible TB using 4-month regimens
- Drug-susceptible TB treatment and ART in people living with HIV
- The use of adjuvant steroids in the treatment of TB meningitis and pericarditis
▶
▶
▶
▶
- Research priorities
- References
- Annex. Summary of changes in policy on DS-TB treatment since 2010 and mapping of recommendations in consolidated DS-TB guidelines
- Web Annexes
▶
▶
▶
▶
-
Module 4: Drug-resistant tuberculosis treatment
- Abbreviations and acronyms
- Acknowledgements
- Definitions
- Executive summary
- Introduction
-
Recommendations
-
Section 1. The 6-month bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) regimen for MDR/RR-TB (NEW)
- 1.1 Recommendations
- 1.2 Summary of evidence
- 1.3 Evidence to recommendations: considerations
- 1.4 Subgroup considerations
- 1.5 Implementation considerations
- 1.6 Monitoring and evaluation
▶
▶
▶
▶
-
Section 2. The 9-month all-oral regimen for MDR/RR-TB (NEW)
- 2.1 Recommendation
- 2.2 Summary of evidence
- 2.3 Evidence to recommendations: considerations
- 2.4 Subgroup considerations
- 2.5 Implementation considerations
- 2.6 Monitoring and evaluation
▶
▶
▶
▶
-
Section 3. Longer regimens for MDR/RR-TB
- 3.1 Recommendations
- 3.2 Justification and evidence
- 3.3 Remarks
- 3.4 Subgroup considerations
- 3.5 Implementation considerations
- 3.6 Monitoring and evaluation
▶
▶
▶
▶
-
Section 4. Regimen for rifampicin-susceptible, isoniazid-resistant TB (Hr-TB)
- 4.1 Recommendations
- 4.2 Justification and evidence
- 4.3 Subgroup considerations
- 4.4 Implementation considerations
- 4.5 Monitoring and evaluation
▶
▶
▶
▶
-
Section 5. Monitoring patient response to MDR/RR-TB treatment using culture
- 5.1 Recommendation
- 5.2 Justification and evidence
- 5.3 Subgroup considerations
- 5.4 Implementation considerations
- 5.5 Monitoring and evaluation
▶
▶
▶
▶
-
Section 6. Starting antiretroviral therapy in patients on MDR/RR-TB regimens
- 6.1 Recommendation
- 6.2 Justification and evidence
- 6.3 Summary of findings
- 6.4 Benefits
- 6.5 Risks
- 6.6 Values and preferences
▶
▶
▶
▶
-
Section 7. Surgery for patients on MDR/RR-TB treatment
- 7.1 Recommendation
- 7.2 Justification and evidence
- 7.3 Subgroup considerations
- 7.4 Implementation considerations
- 7.5 Monitoring and evaluation
▶
▶
▶
▶
▶
▶
▶
▶
-
Section 1. The 6-month bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) regimen for MDR/RR-TB (NEW)
-
Research gaps
- Section 1. The 6-month BPaLM regimen for treatment of MDR/RR-TB or pre-XDR-TB
- Section 2. The 9-month all-oral regimen for MDR/RR-TB
- Section 3. Longer regimens for MDR/RR-TB
- Section 4. Regimens for rifampicin-susceptible, isoniazid-resistant TB (Hr-TB)
- Section 5. Monitoring patient response to MDR/RR-TB treatment using culture
- Section 6. Starting antiretroviral therapy in patients on MDR/RR-TB regimens
- Section 7. Surgery for patients on MDR/RR-TB treatment
▶
▶
▶
▶
- References
- Annex 1. Supplementary table
- Annex 2. Trial population in ZeNix and TB-PRACTECAL trials
- Web Annexes
▶
▶
▶
▶
-
Module 4: Tuberculosis care and support
- Acknowledgements
- List of abbreviations
- Executive summary
- Introduction
-
WHO policy recommendations
- 1. Care and support interventions for all people with TB
- 2. Models of care for people with drug-resistant TB
- 3. Models of care for children and adolescents exposed to TB or with TB disease
▶
▶
▶
▶
- Research priorities
- References
-
Annexes
▶
▶
▶
▶
- Web Annexes
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 4: Drug-susceptible tuberculosis treatment
-
Module 5: Children and adolescents
-
Module 5: Management of tuberculosis in children and adolescents
- Acknowledgements
- Abbreviations
- Definitions
- Executive summary
-
1. Introduction
- 1.1. Background
- 1.2. Rationale for the development of the 2022 consolidated guidelines
- 1.3. Objectives of the 2022 consolidated guidelines
- 1.4. Target audience
- 1.5. WHO recommendations relevant to the management of TB in children and adolescents
-
1.6. Scope of the guideline update
▶
▶
▶
▶
- 1.7. Publication, dissemination, evaluation and expiry
- 1.8. Document structure
▶
▶
▶
▶
- 2. TB screening and contact investigation
- 3. Prevention of TB
-
4. Diagnostic approaches for TB in children and adolescents
-
4.1. The use of the Xpert MTB/RIF Ultra assay in gastric aspirate and stool specimens for the diagnosis of pulmonary TB and rifampicin resistance
- 4.1.1. Justification and evidence
- 4.1.2. Subgroup considerations
- 4.1.3. Implementation considerations
- 4.1.4. Monitoring and evaluation
▶
▶
▶
▶
-
4.2. Treatment decision algorithms for the diagnosis of pulmonary TB in children aged below 10 years of age
- 4.2.1. Justification and evidence
- 4.2.2. Subgroup considerations
- 4.2.3. Implementation considerations
- 4.2.4. Monitoring and evaluation
▶
▶
▶
▶
- 4.3. Consolidated recommendations on TB diagnostics and diagnostic approaches relevant to children and adolescents
▶
▶
▶
▶
-
4.1. The use of the Xpert MTB/RIF Ultra assay in gastric aspirate and stool specimens for the diagnosis of pulmonary TB and rifampicin resistance
-
5. Treatment of TB disease in children and adolescents
-
5.1. Treatment shortening in children and adolescents with non-severe TB
- 5.1.1. Justification and evidence
- 5.1.2. Subgroup considerations
- 5.1.3. Implementation considerations
- 5.1.4. Monitoring and evaluation
▶
▶
▶
▶
-
5.2. Treatment regimens for TB meningitis in children and adolescents
- 5.2.1. Justification and evidence
- 5.2.2. Subgroup considerations
- 5.2.3. Implementation considerations
- 5.2.4. Monitoring and evaluation
▶
▶
▶
▶
-
5.3. Treatment of multi-drug and rifampicin resistant TB in children
- 5.3.1. The use of bedaquiline in children with MDR/RR-TB aged below 6 years
- 5.3.2. The use of delamanid in children with MDR/RR-TB aged below 3 years
▶
▶
▶
▶
- 5.4. Consolidated recommendations on TB treatment for children and adolescents
▶
▶
▶
▶
-
5.1. Treatment shortening in children and adolescents with non-severe TB
-
6. Models of TB care for case detection and provision of TPT in children and adolescents
-
6.1. Decentralized and family-centred, integrated models of care to deliver child and adolescent TB services
- 6.1.1. Justification and evidence
- 6.1.2. Subgroup considerations
- 6.1.3. Implementation considerations
- 6.1.4. Monitoring and evaluation
▶
▶
▶
▶
- 6.2. Consolidated recommendations on models of TB care relevant to children and adolescents
▶
▶
▶
▶
-
6.1. Decentralized and family-centred, integrated models of care to deliver child and adolescent TB services
- 7. Special situations
- 8. Research priorities
- 9. References
- Annex 1. WHO recommendations incorporated in the guidelines on the management of TB in children and adolescents
- Annex 2. Supplementary table
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 5: Management of tuberculosis in children and adolescents
-
Module 6: Comorbidities
-
Module 6: Nutritional care and support for TB patients
- Acknowledgements
- Financial support
- Abbreviations
- Executive summary
- 1. Scope and purpose
- 2. Background
- 3. Guideline development process
- 4. Summary of the evidence
- 5. Key principles
- 6. Recommendations
- 7. Dissemination, adaptation and implementation
- 8. Plans for updating the guideline
- References
- Annex 1 GRADE summary of findings tables
- Annex 2 Summary of the Nutrition Guidance Advisory Group’s considerations for determining the strength of the recommendation
- Annex 3 Questions in population, intervention, control, outcomes (PICO) format
- Annex 4 WHO Steering Committee for Nutrition Guidelines Development 2010–2011
- Annex 5 Nutrition Guidance Advisory Group – nutrition in the life-course 2010–2011, WHO Secretariat and external resource experts
- Annex 6 External experts’ and stakeholders’ panel
▶
▶
▶
▶
-
Module 6: Joint WHO/ILO policy guidelines on improving health worker access to prevention, treatment and care services for HIV and TB
- Abbreviations, Acronyms and Selected Definitions
- Executive Summary
-
1. Introduction
-
1.1 Rationale and Objectives
▶
▶
▶
▶
-
1.2 Policy Formulation Process
- 1.2.1 Preliminary Review and Methodological Decisions
- 1.2.2 Methods for the Corbett Study
- 1.2.3 Methods for National Survey Conducted by the Guideline Group
- 1.2.4 Methods for the Cochrane-Style Systematic Review
- 1.2.5 Evidence Grading and Formulation of Recommendations
▶
▶
▶
▶
- 1.3 Values
▶
▶
▶
▶
-
1.1 Rationale and Objectives
-
2. Statements and Recommendations
-
2.1 Statement #1
- 2.1.1 Introduce new, or refine existing, national policies that ensure priority access for health workers and their families to services for the prevention, treatment and care for HIV and TB.
- 2.1.2 Key References and Supporting WHO Guidelines
- 2.1.3 Table 3: Recommendation for Statement 1
▶
▶
▶
▶
-
2.2 Statement #2
- 2.2.1 Introduce new, or reinforce existing, policies that prevent discrimination against health workers with HIV or TB, and adopt interventions aimed at stigma reduction among colleagues and supervisors.
- 2.2.2 Key References and Supporting WHO Guidelines
- 2.2.3 Table 4: Recommendation for Statement 2
▶
▶
▶
▶
-
2.3 Statement #3
- 2.3.1 Develop or strengthen existing occupational health services for the entire health workforce so that access to HIV and TB prevention, treatment and care can be realized.
- 2.3.2 Key References and Supporting WHO Guidelines
- 2.3.3 Table 5: Recommendation for Statement 3
▶
▶
▶
▶
-
2.4 Statement #4
- 2.4.1 Develop or strengthen existing infection control programmes, especially with respect to TB infection control, and ensure integration with other workplace health and safety programmes.
- 2.4.2 Key References and Supporting WHO Guidelines
- 2.4.3 Table 6: Recommendation for Statement 4
▶
▶
▶
▶
-
2.5 Statement #5
- 2.5.1 In conjunction with health workers’ representatives, develop and implement programmes for regular, free, voluntary, and confidential counselling and testing for HIV and TB, including addressing sexual and reproductive health issues, as well as intensified case finding in the families of health workers with TB.
- 2.5.2 Key References and Supporting WHO Guidelines
- 2.5.3 Table 7: Recommendation for Statement 5
▶
▶
▶
▶
-
2.6 Statement #6
- 2.6.1 Develop and implement training programmes for pre-service, in-service and continuing education on TB and HIV prevention, treatment and care services, integrating with existing programmes and including managers and worker representatives as well as health workers.
- 2.6.2 Key References and Supporting WHO Guidelines
- 2.6.3 Table 8: Recommendation for Statement 6
▶
▶
▶
▶
-
2.7 Statement #7
- 2.7.1 Disseminate policies in the form of guidelines and codes of practices for application at the level of health facilities, and ensure provision of budgets for the training and material inputs to make them operational.
- 2.7.2 Key References and Supporting WHO Guidelines
- 2.7.3 Table 9: Recommendation for Statement 7
▶
▶
▶
▶
-
2.8 Statement #8
- 2.8.1 Adapt and implement good practices in occupational health and the management of HIV and TB in the workplace from all sectors.
- 2.8.2 Key References and Supporting WHO Guidelines
- 2.8.3 Table 12: Recommendation for Statement 8
▶
▶
▶
▶
-
2.9 Statement #9
- 2.9.1 Establish and provide adequate financial resources for treatment, care and support programmes to prevent the occupational or non- occupational transmission of HIV and TB among health workers.
- 2.9.2 Key References and Supporting WHO Guidelines
- 2.9.3 Table 13: Recommendation for Statement 9
▶
▶
▶
▶
-
2.10 Statement #10
- 2.10.1 Provide universal availability of free and timely PEP to all health care providers, for both occupational and non-occupational exposures, with appropriate training of counsellors and information on the benefits and risks provided to all staff.
- 2.10.2 Key References and Supporting WHO Guidelines
- 2.10.3 Table 14: Recommendation for Statement 10
▶
▶
▶
▶
-
2.11 Statement #11
- 2.11.1 Provide free HIV and TB treatment for health workers in need, facilitating the delivery of these services in a non-stigmatizing, gendersensitive, confidential, and convenient setting even where there is no staff clinic, and/or the health worker’s own facility does not offer ART.
- 2.11.2 Key References and Supporting WHO Guidelines
- 2.11.3 Table 15: Recommendation for Statement 11
▶
▶
▶
▶
-
2.12 Statement #12
- 2.12.1 In the context of preventing co-morbidity, provide universal availability of a comprehensive package of prevention and care for all HIV positive health workers, including IPT and CTX prophylaxis, with appropriate information on the benefits and risks
- 2.12.2 Key References and Supporting WHO Guidelines
- 2.12.3 Table 16: Recommendation for Statement 12
▶
▶
▶
▶
-
2.13 Statement #13
- 2.13.1 Establish schemes for reasonable accommodation and compensation, including, as appropriate, paid leave, early retirement benefits and death benefits in the event of occupationally-acquired disease.
- 2.13.2 Key References and Supporting WHO Guidelines
- 2.13.3 Table 17: Recommendation for Statement 13
▶
▶
▶
▶
-
2.14 Statement #14
- 2.14.1 Develop and implement mechanisms for monitoring the availability of these TREAT policy guidelines at the national level, as well as the dissemination of these policies and their application in the healthcare setting.
- 2.14.2 Key References and Supporting ILO and WHO Guidelines
- 2.14.3 Table 18: Recommendation for Statement 14
▶
▶
▶
▶
▶
▶
▶
▶
-
2.1 Statement #1
-
3. Integrating framework and implementation plan
▶
▶
▶
▶
- References
- Annex 1: WHO and other international guidelines referenced
- Annex 2: Follow-up and implementation; an Extract from the Report: International consultation policy guidelines on improving health workers' access to prevention, treatment and care services for HIV and TB 14-16 September 2009, WHO/Geneva (pp 41-46)
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 6: Nutritional care and support for TB patients
▶
▶
-
Module 1: Prevention
-
Operational Handbooks
-
Module 1: Prevention
-
Module 1: TB preventive treatment
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- 1. Introduction
- 2. Identifying populations for TB preventive treatment
- 3. Screening for TB and ruling out TB disease before TB preventive treatment
- 4. Testing for TB infection
-
5. TB preventive treatment
▶
▶
▶
▶
-
6. Safety and management of adverse drug reactions in TB preventive treatment
▶
▶
▶
▶
-
7. Supporting people in adhering to and completing TB preventive treatment
▶
▶
▶
▶
- 8. Monitoring and evaluation
- 9. Ethics and TB preventive treatment
- References
-
Annexes
- Annex 1. Investment case for TB screening and preventive treatment
- Annex 2. Messages for different stakeholders
- Annex 3. Coordination mechanisms to support PMTPT
- Annex 4. Costing considerations for PMTPT
- Annex 5. Checklist for PMTPT components in reviews of national programmes
- Annex 6. Variables to be collected for TB contact evaluation
▶
▶
▶
▶
-
Web annexes
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 1: TB laboratory biosafety
- Executive summary
- Participants in the guideline development process
-
Abbreviations
▶
▶
▶
▶
- Introduction
-
1. Risk assessment and the classification of tb laboratories
- 1.1 Risk assessment for tb laboratories: what is it?
- 1.2 Hazard identification
- 1.3 Determining risks
- 1.4 Monitoring risks and mitigation measures
- 1.5 Employee occupational health programme
- 1.6 Classification of tb laboratories
▶
▶
▶
▶
-
2. Essential biosafety measures for tb laboratories
-
2.1 Codes of practice
- 2.1.1 Laboratory access
- 2.1.2 Responsibilities of the laboratory manager
- 2.1.3 Personal protective equipment
- 2.1.4 Procedures
- 2.1.5 Work areas
▶
▶
▶
▶
- 2.2 Equipment
- 2.3 Design and facilities
- 2.4 Training
-
2.5 Waste handling
▶
▶
▶
▶
-
2.6 Disposal procedures for contaminated materials
- 2.6.1 Broken glass and glass slides
- 2.6.2 Contaminated or potentially infectious materials for disposal
▶
▶
▶
▶
▶
▶
▶
▶
-
2.1 Codes of practice
-
3. Low-risk tb laboratories
- 3.1 Factors that increase the risk of infection
- 3.2 Specific features and essential minimum biosafety measures
▶
▶
▶
▶
-
4. Moderate-risk tb laboratories
- 4.1 Factors that increase the risk of infection
- 4.2 Specific features and essential minimum biosafety measures
▶
▶
▶
▶
-
5. High-risk tb laboratories (tb-containment laboratories)
- 5.1 Factors that increase the risk of infection
- 5.2 Specific features and required biosafety measures
▶
▶
▶
▶
-
6. Safety equipment
-
6.1 Biological safety cabinets
- 6.1.1 Selecting a biological safety cabinet for a TB laboratory
- 6.1.2 Class I biological safety cabinets
- 6.1.3 Class II type A2 biological safety cabinets
- 6.1.4 Thimble connections
- 6.1.5 Using biological safety cabinets in the laboratory
▶
▶
▶
▶
- 6.2 Centrifuges with safety buckets
- 6.3 Autoclaves
▶
▶
▶
▶
-
6.1 Biological safety cabinets
-
7. Personal protective equipment and clothing
- 7.1 Laboratory gowns
-
7.2 Respirators
▶
▶
▶
▶
-
7.3 Gloves
▶
▶
▶
▶
▶
▶
▶
▶
-
8. Plans for emergency preparedness and response
- 8.1 Emergency preparedness plan
-
8.2 Emergency response procedures for tb laboratories
- 8.2.1 Infectious spills (outside a biological safety cabinet)
- 8.2.2 Infectious spills (contained within a biological safety cabinet)
- 8.2.3 Breakage of tubes inside sealed buckets (safety cups)
▶
▶
▶
▶
- 8.3 Spill clean-up kit
▶
▶
▶
▶
- 9. References
-
10. Annex
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 1: Infection prevention and control
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- 1. Introduction
- 2. Administrative controls
- 3. Environmental controls
- 4. Respiratory protection
- 5. TB IPC in special situations
- 6. Monitoring and evaluation
- References
-
Annexes
- Annex 1. Data elements for monitoring implementation of tuberculosis infection prevention and control
- Annex 2. Facility tuberculosis risk assessment tool
- Annex 3. Example of an outline of facility tuberculosis infection prevention and control plan
- Annex 4. Health care worker tuberculosis screening form
- Annex 5. Health care worker TB screening register
- Annex 6. Sample posters for health education
- Annex 7. How to choose upper-room germicidal ultraviolet light fixtures
- Annex 8. Choosing a radiometer for measurement of ultraviolet C irradiation
- Annex 9. Checklist for the review of programmatic implementation of tuberculosis infection prevention and control
- Annex 10. Country example: education messages for tuberculosis and for tuberculosis infection prevention and control
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 1: TB preventive treatment
-
Module 2: Screening
-
Module 2: Systematic screening for tuberculosis disease
- Acknowledgements
- Abbreviations and acronyms
- Definitions
-
Chapter 1. Introduction
- 1.1 Rationale for systematic screening for TB disease
- 1.2 Principles of TB screening
- 1.3 Objectives of the operational handbook
- 1.4 Target audience of the operational handbook
▶
▶
▶
▶
-
Chapter 2. The six steps in the planning and implementation cycle
-
2.1 Introduction
- 2.1.1 Enhancing the patient-initiated pathway to TB diagnosis
- 2.1.2 The provider-initiated screening pathway to TB diagnosis
▶
▶
▶
▶
-
2.2 Assessing the situation
- 2.2.1 Existing screening and outreach activities
- 2.2.2 Societal context
- 2.2.3 Epidemiology of TB
- 2.2.4 National TB programmes and the general health-care system
- 2.2.5 Health-care coverage and access to health services
- 2.2.6 Protection from stigmatization, discrimination and harm
▶
▶
▶
▶
- 2.3 Setting goals and specific objectives
-
2.4 Identifying and prioritizing risk groups
- 2.4.1 Potential benefits for the individual
- 2.4.2 Potential risks and harms for the individual
- 2.4.3 Potential impact on prevalence and transmission
- 2.4.4 Potential total yield of true TB cases
- 2.4.5 Number needed to screen to detect a person with TB
- 2.4.6 Cost–effectiveness and cost–benefit analyses
▶
▶
▶
▶
- 2.5 Choosing algorithms for screening and diagnosis
-
2.6 Planning, budgeting and implementing
- 2.6.1 Requirements for planning, human resources, commodities and budgeting
- 2.6.2 Choosing a screening programme model
- 2.6.3 Ethical considerations
- 2.6.4 Involving stakeholders and partner organizations and establishing roles
- 2.6.5 Mobilizing resources
- 2.6.6 Pilot-testing
▶
▶
▶
▶
-
2.7 Monitoring, evaluating and modifying the programme
- 2.7.1 Developing a plan for monitoring and evaluation
- 2.7.2 Proposed indicators
- 2.7.3 Routines for recording and reporting
- 2.7.4 Programmatic evaluations
- 2.7.5 Monitoring time trends for re-screening and re-prioritization
▶
▶
▶
▶
▶
▶
▶
▶
-
2.1 Introduction
-
Chapter 3. Screening tools and algorithms
-
3.1 Screening tools
- 3.1.1 Symptom screening
- 3.1.2 CXR screening
- 3.1.3 CAD technologies for screening and triage
- 3.1.4 Molecular WHO-recommended rapid diagnostics for screening
- 3.1.5 Tests of TB infection
▶
▶
▶
▶
-
3.2 Algorithms for screening
- 3.2.1 Basic features of TB screening and diagnostic algorithms
- 3.2.2 Screening and diagnostic algorithm options
- 3.2.3 Choosing an algorithm for a screening programme
▶
▶
▶
▶
- 3.3 ScreenTB tool
▶
▶
▶
▶
-
3.1 Screening tools
-
Chapter 4. Implementation of CAD technologies in a new setting
- 4.1 Considerations in selecting and using CAD for screening in TB programmes
- 4.2 Toolkit for CAD calibration to enable implementation
- 4.3 Online tool for calibration of CAD in a new setting
▶
▶
▶
▶
-
Chapter 5. Screening for tuberculosis disease among adults and adolescents living with HIV
- 5.1 Introduction
-
5.2 Screening tools
- 5.2.1 WHO-recommended four-symptom screen
- 5.2.2 C-reactive protein (CRP)
- 5.2.3 Chest X-ray
- 5.2.4 WHO-recommended rapid molecular diagnostic tests
▶
▶
▶
▶
- 5.3 Considerations for use of all screening tools
- 5.4 Algorithms for screening
▶
▶
▶
▶
-
Chapter 6. Screening for tuberculosis disease in children
- 6.1 Introduction
-
6.2 Screening child contacts of patients with TB
- 6.2.1 Symptom screening
- 6.2.2 CXR
- 6.2.3 WHO-recommended rapid molecular diagnostic tests
- 6.2.4 Tests of TB infection
- 6.2.5 Considerations for implementation
▶
▶
▶
▶
-
6.3 Screening children living with HIV
- 6.3.1 Screening for symptoms and contact
- 6.3.2 Other screening tests
- 6.3.3 Considerations for implementation
▶
▶
▶
▶
- 6.4 Algorithms for screening
▶
▶
▶
▶
- References
-
Annex 1 Screening algorithms for the general population and high-risk groups (not including people living with HIV)
- A.1.1 – Screening with cough
- A.1.2 – Parallel screening with cough and CXR
- A.1.3 – Sequential positive serial screening with cough and CXR
- A.1.4 ‒ Rastreamento seriado negativo sequencial com tosse e radiografia de tórax
- A.1.5 – Screening with any TB symptom
- A.1.6 – Parallel screening with any TB symptom and CXR
- A.1.7 – Sequential positive serial screening with any TB symptom and CXR
- A.1.8 – Sequential negative serial screening with any TB symptom and CXR
- A.1.9 – Screening with CXR
- A.1.10 – Screening with mWRD
▶
▶
▶
▶
- Annex 2 Comparative performance of algorithms for the general population and high-risk groups (not including people living with HIV)
-
Annex 3 Screening algorithms for adults and adolescents living with HIV
- A.3.1 – W4SS single screening algorithm
- A.3.2 – CRP single screening algorithm
- A.3.3 – CXR single screening algorithm
- A.3.4 – Parallel screening algorithm with W4SS and CRP
- A.3.5 – Sequential positive screening algorithm with W4SS and CRP
- A.3.6 – Sequential negative screening algorithm with W4SS and CRP
- A.3.7 – Parallel screening algorithm with W4SS and CXR
- A.3.8 – Sequential positive screening algorithm with W4SS and CXR
- A.3.9 – Sequential negative screening algorithm with W4SS and CXR
- A.3.10 – mWRD single screening algorithm for medical inpatients in settings with TB prevalence > 10%
- A.3.11 – mWRD single screening algorithm for people living with HIV
▶
▶
▶
▶
- Annex 4 Comparative performance of algorithms for adults and adolescents living with HIV
-
Annex 5 Screening algorithms for children
- A.5.1 – Screening with symptoms
- A.5.2 – Screening with CXR
- A.5.3 – Parallel screening with symptoms and CXR
- A.5.4 – Sequential positive serial screening with symptoms and CXR
- A.5.5 – Sequential negative serial screening with symptoms and CXR
- A.5.6 – Screening with symptoms (for children living with HIV < 10 years)
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 2: Systematic screening for tuberculosis disease
-
Module 3: Diagnosis
-
Module 3: Rapid diagnostics for tuberculosis detection
- Acknowledgements
-
Abbreviations and acronyms
▶
▶
▶
▶
-
1. Introduction
▶
▶
▶
▶
-
2. Diagnostic tests with WHO recommendations
- 2.1 Conventional diagnostic tests for the diagnosis of TB
-
2.2 Initial tests for diagnosis of TB with drug-resistance detection
- 2.2.1 Xpert MTB/RIF assay
- 2.2.2 Xpert MTB/RIF Ultra assay
- 2.2.3 Truenat MTB, MTB Plus and MTB-RIF Dx assays
- 2.2.4 Moderate complexity automated NAATs
▶
▶
▶
▶
-
2.3 Initial tests for diagnosis of TB without drug-resistance detection
▶
▶
▶
▶
-
2.4 Follow-on diagnostic tests for detection of additional drug resistance
- 2.4.1 Low complexity automated NAATs for detection of resistance to INH and second-line anti-TB drugs
- 2.4.2 LPAs
- 2.4.3 High complexity reverse hybridization NAAT
- 2.4.4 Targeted NGS tests
▶
▶
▶
▶
- 2.5 Tests WHO recommends against using
-
2.6 Phenotypic and genotypic DST
▶
▶
▶
▶
▶
▶
▶
▶
-
3. Implementing a new diagnostic test
-
3.1 Placement of diagnostic tests in the tiered laboratory network
- 3.1.1 Peripheral level
- 3.1.2 Intermediate level
- 3.1.3 Central level
- 3.1.4 Structure of network and testing packages
▶
▶
▶
▶
- 3.2 Pretest probability and test accuracy considerations
- 3.3 Epidemiologic considerations
- 3.4 Multi-disease platform considerations
-
3.5 Steps and processes for implementing a new diagnostic test
- 3.5.1 Area 1 – Policies, budgeting and planning
- 3.5.2 Area 2 – Regulatory issues
- 3.5.3 Area 3 – Equipment
- 3.5.4 Area 4 – Supply chain
- 3.5.5 Area 5 – Procedures
- 3.5.6 Area 6 – Digital data
- 3.5.7 Area 7 – Quality assurance, control and assessment
- 3.5.8 Area 8 – Recording and reporting
- 3.5.9 Area 9 – Human resource training and competency assessment
- 3.5.10 Area 10 – Monitoring and evaluation
▶
▶
▶
▶
▶
▶
▶
▶
-
3.1 Placement of diagnostic tests in the tiered laboratory network
-
4. Model algorithms
-
4.1 Algorithm 1 – mWRD as the initial diagnostic test for TB
▶
▶
▶
▶
-
4.2 Algorithm 2 – LF-LAM testing to aid in the diagnosis of TB among PLHIV
▶
▶
▶
▶
-
4.3 Algorithm 3 – DST for second-line drugs for people with RR-TB or MDR-TB
▶
▶
▶
▶
-
4.4 Algorithm 4 – mWRD as the initial or follow-on test to detect Hr-TB
▶
▶
▶
▶
-
4.5 Illustrative algorithm combinations
▶
▶
▶
▶
▶
▶
▶
▶
-
4.1 Algorithm 1 – mWRD as the initial diagnostic test for TB
- 6.References
- Annex 1. Budgetary considerations for implementing a new diagnostic test
-
Annex 2: Information sheets on the newly recommended products
- A2.1 Information sheet: Practical considerations for implementation of the Abbott RealTime MTB and Abbott RealTime MTB RIF/INH tests
- A2.2 Information sheet: Practical considerations for implementation of the BD MAX MDR-TB test
- A2.3 Information sheet: Practical considerations for implementation of the Roche cobas MTB and cobas MTB-RIF/INH assays
- A2.4 Information sheet: Practical considerations for implementation of the Bruker-Hain Lifesciences FluoroType MTB and FluoroType MTBDR
- A2.5 Information sheet: Practical considerations for implementation of the Cepheid Xpert MTB/XDR test
- A2.6 Information sheet: Practical considerations for implementation of the Nipro Genoscholar PZA-TB II assay
▶
▶
▶
▶
- Annex 3. Implementation of next-generation sequencing technologies
- Annex 4. Conflict of interest assessment
- Annex 5. Additional resources
▶
▶
▶
▶
-
Module 3: Tests for tuberculosis infection
- Acknowledgements
- Abbreviations and acronyms
-
1. Introduction
- 1.1 Rationale for TB infection testing
- 1.2 TB infection tests that are currently recommended by WHO
- 1.3 The TB infection cascade of care
- 1.4 About this operational handbook
- 1.5 Target audience
▶
▶
▶
▶
-
2. Testing for TB infection and a model algorithm
- 2.1 Who should be tested for TB infection?
- 2.2 Risk of disease in those with positive tests for TB infection
- 2.3 What are the advantages and disadvantages of testing for TB infection?
-
2.4 When is TB infection testing not advised?
- 2.4.1 Prior positive TB infection tests
- 2.4.2 Concomitant or recent vaccines or viral illnesses
- 2.4.3 Clinical work-up of adults to diagnose TB disease or monitoring of the response to treatment
- 2.4.4 History of TST or TBST allergic reactions (but IGRAs may be used)
- 2.4.5 Challenges with blood collection in young children when using IGRAs (but TBST may be used)
▶
▶
▶
▶
- 2.5 An integrated and person-centred model algorithm
▶
▶
▶
▶
-
3. WHO-recommended tests for TB infection
-
3.1 TB infection skin test using tuberculin (TST)
▶
▶
▶
▶
-
3.2 TB infection skin tests using M. tuberculosis-specific antigen
- 3.2.1 Cy-Tb
- 3.2.2 Diaskintest
- 3.2.3 C-TST
- 3.2.4 Interpretation of TBST – criteria for a positive TBST
▶
▶
▶
▶
-
3.3 Steps and processes for skin testing
- 3.3.1 Materials and supplies needed
- 3.3.2 Personnel needed
- 3.3.3 Training and quality control
- 3.3.4 Overview of test procedures and management
▶
▶
▶
▶
-
3.4 Interferon-gamma release assays
▶
▶
▶
▶
▶
▶
▶
▶
-
3.1 TB infection skin test using tuberculin (TST)
-
4. Programmatic considerations for implementing TB infection tests
- 4.1 General considerations: integrated and person-centred TB infection cascade of care
-
4.2 Steps for programmatic implementation of testing for TB infection
- 4.2.1 Area 1 – Policies, budgeting and planning
- 4.2.2 Area 2 – Regulatory approval and importation of products
- 4.2.3 Area 3 – Equipment and materials
- 4.2.4 Area 4 – Human resource sensitization, training and competency assessment
- 4.2.5 Area 5 – Supply chain
- 4.2.6 Area 6 – Procedures
- 4.2.7 Area 7 – Digital data
- 4.2.8 Area 8 – QA, QC and quality assessment
- 4.2.9 Area 9 – Recording and reporting
- 4.2.10 Area 10 – Monitoring and evaluation
▶
▶
▶
▶
▶
▶
▶
▶
- References
-
Annex 1. Skin tests for tuberculosis infection – detailed description
▶
▶
▶
▶
-
Annex 2. Interferon-gamma release assays – detailed description
- A2.1 QFT-Plus – step by step
- A2.2 WANTAI TB-IGRA – step by step
- A2.3 T-SPOT.TB – step by step
- References for Annex 2
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 3: Rapid diagnostics for tuberculosis detection
-
Module 4: Treatment
-
Module 4: Drug-susceptible tuberculosis treatment
- Acknowledgements
- Abbreviations and acronyms
- Definitions
- Executive summary
-
Key WHO recommendations on DS-TB
- Treatment of DS-TB using the 6-month regimen
- Treatment of DS-TB using 4-month regimens
- DS-TB treatment and antiretroviral therapy in people living with HIV
- The use of adjuvant steroids in the treatment of tuberculous meningitis and pericarditis
▶
▶
▶
▶
- 1. Introduction
-
2. Key considerations in DS-TB treatment
- 2.1 TB diagnostics and drug susceptibility testing
- 2.2 Care and support during TB treatment
- 2.3 Options in treatment of DS-TB
▶
▶
▶
▶
-
3. Treatment of DS-TB using the 6-month regimen
- 3.1 Eligibility
- 3.2 Composition and duration of the regimen 2HRZE/4HR
- 3.3 Considerations for implementation
- 3.4 Subgroups
- 3.5 Treatment monitoring
▶
▶
▶
▶
-
4. Treatment of DS-TB using the 4-month 2HPMZ/2HPM regimen
- 4.1 Eligibility
- 4.2 Composition and duration of the regimen
- 4.3 Considerations for implementation
- 4.4 Subgroups
- 4.5 Treatment monitoring
▶
▶
▶
▶
-
5. Treatment of DS-TB using the 4-month 2HRZ(E)/2HR regimen
- 5.1 Eligibility
- 5.2 Composition and duration of the regimen
- 5.3 Considerations for implementation
- 5.4 Subgroups
- 5.5 Treatment monitoring
▶
▶
▶
▶
-
6. Treatment of DS-TB in People with HIV
- 6.1 Eligibility
- 6.2 Composition and duration of the regimen
- 6.3 Considerations for implementation
- 6.4 Treatment monitoring
▶
▶
▶
▶
-
7. Treatment of extrapulmonary TB
- 7.1 Eligibility
- 7.2 Composition and duration of the regimen
- 7.3 Use of adjuvant steroids in the treatment of tuberculous meningitis and pericarditis
- 7.4 Considerations for implementation
▶
▶
▶
▶
-
8. Treatment of DS-TB in special situations
▶
▶
▶
▶
-
9. Monitoring treatment response
- 9.1 Clinical examination
- 9.2 Chest radiography
- 9.3 Sputum smear and culture
- 9.4 Assessment of patients when treatment failure is suspected
▶
▶
▶
▶
-
10. Outcome definitions
▶
▶
▶
▶
- References
- Annex. Dosages of anti-TB medicines by weight band for treatment of DS-TB
- Web annexes
▶
▶
▶
▶
-
Module 4: Drug-resistant tuberculosis treatment, 2022 update
- Abbreviations
- Acknowledgements
- 1. Introduction
- 2. Commonly used terms and key definitions in DR-TB treatment
-
3. Key considerations in DR-TB treatment
- 3.1 Access to DST
- 3.2 Safety monitoring and management, provision of patient support and management of comorbidities
- 3.3 Regimen options in the treatment of DR-TB
▶
▶
▶
▶
-
4. The 6-month bedaquiline, pretomanid, linezolid and moxifloxacin (BPaLM) regimen
- 4.1 Eligibility
-
4.2 Composition and duration of the regimen
- 4.2.1 BPaLM regimen
- 4.2.2 BPaL regimen
- 4.2.3 Duration of treatment
- 4.2.4 Linezolid dosing in the BPaLM/BPaL regimen
- 4.2.5 Modification of treatment
- 4.2.6 Discontinuation of the regimen
- 4.2.7 Pregnancy during treatment
▶
▶
▶
▶
-
4.3 Key subgroups
- 4.3.1 Children
- 4.3.2 People with HIV
- 4.3.3 Pulmonary TB
- 4.3.4 Extrapulmonary TB
- 4.3.5 Pregnant and breastfeeding women
▶
▶
▶
▶
-
4.4 Implementation considerations
- 4.4.1 DST
- 4.4.2 Treatment support
- 4.4.3 Contact tracing of household contacts
- 4.4.4 Infection control
- 4.4.5 Cost–effectiveness analysis
▶
▶
▶
▶
-
4.5 Treatment monitoring
▶
▶
▶
▶
▶
▶
▶
▶
-
5. The 9-month all-oral regimen
-
5.1 Eligibility
- 5.1.1 Assessment of extent and severity of TB disease
- 5.1.2 DST results
- 5.1.3 Haematological assessment
▶
▶
▶
▶
-
5.2 Composition and duration of the regimen
- 5.2.1 Ethionamide variation
- 5.2.2 Linezolid variation
- 5.2.3 Choice of fluoroquinolone
- 5.2.4 Drug dosage and frequency
- 5.2.5 Regimen modifications
- 5.2.6 Switching between treatment regimens
▶
▶
▶
▶
-
5.3 Key subgroups
- 5.3.1 People with HIV
- 5.3.2 Children
- 5.3.3 Pregnant and breastfeeding women
- 5.3.4 Extensive TB disease
- 5.3.5 Extrapulmonary TB
▶
▶
▶
▶
-
5.4 Implementation considerations and treatment in special situations
▶
▶
▶
▶
-
5.5 Treatment monitoring
- 5.5.1 Monitoring treatment response and outcome assignment
- 5.5.2 Monitoring safety
- 5.5.3 Modification or discontinuation of treatment
▶
▶
▶
▶
-
5.6 Using modified all-oral shorter MDR-TB regimens under operational research
▶
▶
▶
▶
▶
▶
▶
▶
-
5.1 Eligibility
-
6. The longer regimens
- 6.1 Eligibility
-
6.2 Composition and duration of the regimens
- 6.2.1 Choice of components for the longer MDR-TB regimens
- 6.2.2 Medicines used in longer MDR-TB treatment regimens
- 6.2.3 Duration of the regimen
▶
▶
▶
▶
-
6.3 Key subgroups
- 6.3.1 People with HIV
- 6.3.2 Children
- 6.3.3 Pregnant and lactating women
- 6.3.4 Patients with diabetes mellitus
- 6.3.5 Patients with extrapulmonary TB
▶
▶
▶
▶
-
6.4 Implementation considerations and treatment in special situations
- 6.4.1 Extensive DR-TB disease
- 6.4.2 Severe extrapulmonary TB
- 6.4.3 DR-TB in different patient groups
▶
▶
▶
▶
-
6.5 Treatment monitoring
▶
▶
▶
▶
▶
▶
▶
▶
-
7. Regimen for rifampicin susceptible and isoniazid resistant TB
- 7.1 Eligibility
- 7.2 Composition and duration of the regimen
- 7.3 Considerations for implementation
- 7.4 Treatment monitoring
▶
▶
▶
▶
-
8. Adjuncts to MDR-TB treatment
- 8.1 Surgery in the treatment of MDR/XDR-TB
- 8.2 Use of corticosteroids
- 8.3 Treatment of MDR/RR-TB patients with HIV
▶
▶
▶
▶
-
9. Programmatic implementation of MDR-TB regimens
- 9.1 Policy and operational documents
- 9.2 National MDR-TB expert committee or technical working group
- 9.3 Electronic recording and reporting
- 9.4 Estimates (epidemiological and logistics)
- 9.5 Management of the supply chain and storage conditions for pharmaceuticals
- 9.6 Preparation for the introduction of new treatment regimens
▶
▶
▶
▶
-
10. Treatment outcome definitions
▶
▶
▶
▶
- References
- Annex
▶
▶
▶
▶
-
Module 4: Tuberculosis care and support
- Acknowledgements
- Abbreviations
- Definitions
- 1. Introduction
- 2. People-centred approach
-
3. Care and support interventions to enable TB treatment adherence
-
3.1. Social support in TB management
- 3.1.1 Informational and educational support
- 3.1.2 Psychological and emotional support
- 3.1.3 Material support
- 3.1.4 Companionship support
▶
▶
▶
▶
-
3.2. Treatment administration and digital adherence technologies
▶
▶
▶
▶
- 3.3. Selecting a suitable package of care and support for a patient
▶
▶
▶
▶
-
3.1. Social support in TB management
-
4. Health education and counselling for people affected with tuberculosis
- 4.1. Guiding principles for health education and counselling
-
4.2. Effective communication skills to provide health education and counselling
- 4.2.1 Forming a therapeutic alliance
- 4.2.2 Active listening
- 4.2.3 Using non-verbal communication
- 4.2.4 Asking questions
- 4.2.5 Providing information
▶
▶
▶
▶
-
4.3. Counselling to provide information about TB and the responsibilities of affected individuals and communities
▶
▶
▶
▶
-
4.4. Counselling to provide information about TB treatment and to ensure adherence to treatment
- 4.4.1 Counselling to provide information about TB treatment
- 4.4.2 Counselling to ensure adherence to treatment
▶
▶
▶
▶
-
4.5. Counselling to provide psychological support
- 4.5.1 Basic psychological support
- 4.5.2 Strengthen social support
- 4.5.3 Problem-solving technique
- 4.5.4 Provide support to the caregivers
- 4.5.5 Refer to mental health services
▶
▶
▶
▶
-
4.6. Counselling on nutritional care and support
- 4.6.1 Treat the underlying cause
- 4.6.2 Educate the person
- 4.6.3 Assess ‘readiness’ of the person to change diet/lifestyle
- 4.6.4 Motivate the person
- 4.6.5 Rewarding desired behaviour (in children)
▶
▶
▶
▶
-
4.7. Counselling at the end of TB treatment and on palliative care
- 4.7.1 Counselling at the end of TB treatment and post-TB treatment
- 4.7.2 Counselling on palliative care
▶
▶
▶
▶
▶
▶
▶
▶
-
5. Models of care for TB services
-
5.1. Models of care for all TB patients
- 5.1.1 Outpatient model of TB treatment: decentralized care
- 5.1.2 Inpatient model of TB treatment and care
- 5.1.3 Deciding on the best-suited model for a situation
▶
▶
▶
▶
- 5.2. Decentralized and integrated family-centred models of TB care for children and adolescents
- 5.3. Models of service delivery for people with TB, HIV and comorbidities
- 5.4. Private-sector involvement in TB care
- 5.5. TB and health emergencies
▶
▶
▶
▶
-
5.1. Models of care for all TB patients
-
6. Palliative care
-
6.1 What is palliative care?
- 6.1.1 Why is palliative care an essential part of comprehensive TB care?
- 6.1.2 When and where should palliative care be provided for people with TB?
- 6.1.3 Who should provide palliative care for people with TB?
- 6.1.4 What is end-of-life care for people with TB?
▶
▶
▶
▶
-
6.2. Planning and implementing palliative care for people affected by TB
- 6.2.1 Integration of palliative care into national tuberculosis programmes
- 6.2.2 Essential package of palliative care for people affected by TB
- 6.2.3 Oxygen for relief of mild dyspnoea
- 6.2.4 Morphine for safe relief of chronic or refractory dyspnoea
- 6.2.5 Palliative care teamwork
- 6.2.6 Management of substance use disorders and other comorbidities
- 6.2.7 Monitoring and evaluation of palliative care for people affected by TB
- 6.2.8 Cost savings from palliative care integration into TB programmes
▶
▶
▶
▶
-
6.3. End-of-life care for people with TB
- 6.3.1 When should suspension of TB treatment be considered?
- 6.3.2 Important considerations in suspending TB treatment
- 6.3.3 Decision-making about suspension of TB treatment
- 6.3.4 Providing end-of-life care for people with TB
▶
▶
▶
▶
▶
▶
▶
▶
-
6.1 What is palliative care?
- References
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 4: Drug-susceptible tuberculosis treatment
-
Module 5: Children and adolescents
-
Module 5: Management of tuberculosis in children and adolescents
- Acknowledgments
- Abbreviations
- Definitions
-
1. Introduction
- 1.1 Background
- 1.2 Children and adolescents as a key vulnerable population
- 1.3 Rationale and objectives of this operational handbook
- 1.4. Preferences of end-users regarding content and structure of this operational handbook
- 1.5. Structure of the operational handbook
- 1.6 Target audience
▶
▶
▶
▶
-
2. TB screening and contact investigation
- 2.1 Introduction
-
2.2 Contact investigation
- 2.2.1 Prioritizing household contacts
- 2.2.2 Planning and budgeting to implement or strengthen household contact investigation
- 2.2.3 Key implementation steps in contact investigation
- 2.2.4 Examples of facility- and community-based approaches for TB contact investigation
▶
▶
▶
▶
-
2.3 TB screening approaches in children and adolescents
-
2.3.1. Screening child contacts of people with bacteriologically confirmed TB
- 2.3.1.1. Symptom screening
- 2.3.1.2. Chest X-ray
- 2.3.1.3. Molecular WHO-recommended rapid diagnostic tests
- 2.3.1.4. Tests for TB infection
▶
▶
▶
▶
- 2.3.2. Considerations for implementation of screening of child close contacts
▶
▶
▶
▶
-
2.3.1. Screening child contacts of people with bacteriologically confirmed TB
-
2.4 Screening children and adolescents living with HIV
- 2.4.1. Screening for symptoms and contact
- 2.4.2. Other screening tests
- 2.4.3. Considerations for implementation
- 2.4.4. Screening of adolescents living with HIV
▶
▶
▶
▶
- Key messages
▶
▶
▶
▶
-
3. Prevention of TB in children and adolescents
- 3.1 Introduction
-
3.2 BCG vaccination
-
3.2.1. Recommendations from the WHO BCG position paper
- 3.2.1.1. Children living with HIV and neonates
- 3.2.1.2. Administering BCG
- 3.2.1.3. Contraindications for BCG
- 3.2.1.4. Adverse reactions
▶
▶
▶
▶
▶
▶
▶
▶
-
3.2.1. Recommendations from the WHO BCG position paper
-
3.3 TB preventive treatment
- 3.3.1. Introduction
-
3.3.2. Target groups for TB preventive treatment
▶
▶
▶
▶
-
3.3.3. Ruling out TB disease before starting TB preventive treatment
- 3.3.3.1. HIV-negative household and close contacts of a person with pulmonary TB: infants and children aged under 5 years
- 3.3.3.2. HIV-negative household and close contacts of a person with pulmonary TB: children and adolescents aged 5 years and over
- 3.3.3.3. Children and adolescents living with HIV
▶
▶
▶
▶
-
3.3.4. Testing for TB infection
▶
▶
▶
▶
-
3.3.5. Options for TB preventive treatment regimens: drug-susceptible TB
▶
▶
▶
▶
- 3.3.6. Options for TB preventive treatment regimens: drug-resistant TB
- 3.3.7. Follow-up of children and adolescents on TB preventive treatment
-
3.3.8. Adherence to TB preventive treatment
▶
▶
▶
▶
- 3.3.9. Other issues related to TB preventive treatment in children and adolescents
▶
▶
▶
▶
- 3.4 TB infection prevention and control
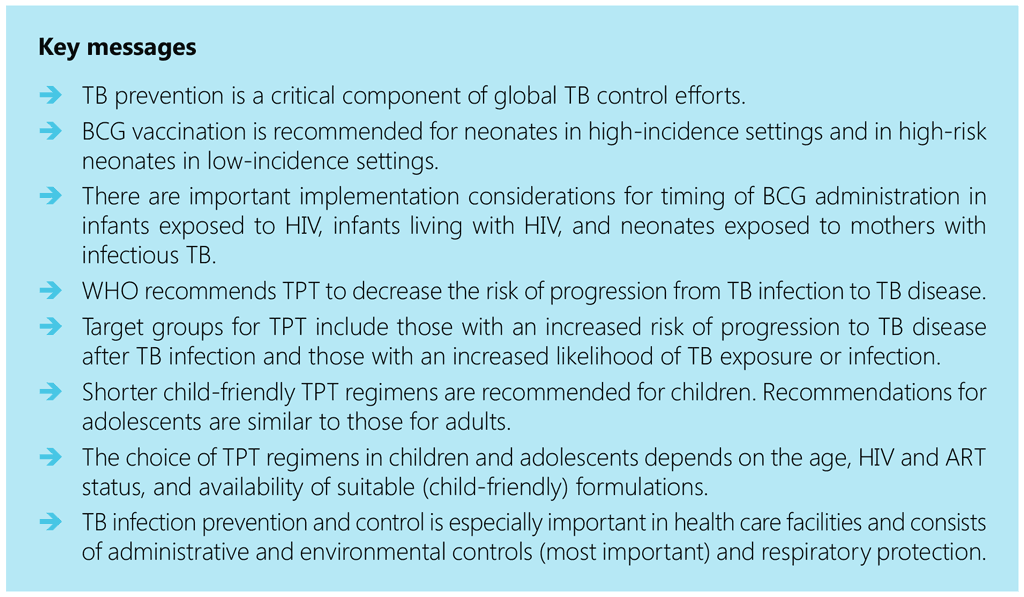
- Key messages
▶
▶
▶
▶
-
4. TB diagnostic approaches for children and adolescents
- 4.1 Introduction
- 4.2 Diagnosing TB in children and adolescents
-
4.3 Diagnostic approaches: pulmonary TB
- 4.3.1. Typical symptoms of pulmonary TB
- 4.3.2. History of TB contact
- 4.3.3. Clinical examination
- 4.3.4. Atypical clinical presentations of children with pulmonary TB
-
4.3.5. Bacteriological confirmation
- 4.3.5.1. Sample types
- 4.3.5.2. WHO-recommended rapid diagnostic tests
- 4.3.5.3. Molecular WHO-recommended rapid diagnostic tests for TB
- 4.3.5.4. Antigen detection in a lateral flow format (biomarker-based detection)
- 4.3.5.5. Repeat testing with molecular WHO-recommended rapid diagnostic tests
▶
▶
▶
▶
- 4.3.6. Testing for TB infection
- 4.3.7. Role of chest X-ray
- 4.3.8. HIV testing
-
4.3.9. Integrated treatment decision algorithms for pulmonary TB in children
- 4.3.9.1. Algorithm A (for settings with chest X-ray) and Algorithm B (for settings without chest X-ray)
- 4.3.9.2. Using the integrated treatment decision algorithms
▶
▶
▶
▶
▶
▶
▶
▶
- 4.4 Diagnostic approaches: extrapulmonary TB
- 4.5 Disease severity
- 4.6 Diagnostic approaches: drug-resistant TB
- Key messages
▶
▶
▶
▶
-
5. Treatment of drug-susceptible and drug-resistant pulmonary and extrapulmonary TB in children and adolescents
- 5.1 Introduction
-
5.2 Treatment of drug-susceptible TB in children and adolescents
- 5.2.1. Principles of TB management
- 5.2.2. Treatment of pulmonary TB in children and adolescents
- 5.2.3. Recommended regimens for treatment of drug susceptible pulmonary TB in children
-
5.2.4. Implementation considerations
- 5.2.4.1. Assessing eligibility for the 4-month regimen
- 5.2.4.2. Inclusion of ethambutol in the intensive phase of treatment
- 5.2.4.3. Implementation considerations for the isoniazid, rifapentine, moxifloxacin and pyrazinamide regimen
- 5.2.4.4. Dosing frequency
▶
▶
▶
▶
-
5.2.5. Subgroup considerations
- 5.2.5.1. Children with peripheral lymph node TB
- 5.2.5.2. Children and adolescents living with HIV
- 5.2.5.3. Children with severe acute malnutrition
- 5.2.5.4. Children with severe acute pneumonia
- 5.2.5.5. Infants aged under 3 months or weighing less than 3 kg
- 5.2.5.6. Children and adolescents treated for TB in past 2 years
- 5.2.5.7. Children and young adolescents with severe pulmonary TB
▶
▶
▶
▶
-
5.2.6. Treatment of drug-susceptible extrapulmonary TB in children and adolescents
- 5.2.6.1. Treatment of TB meningitis and osteoarticular TB
- 5.2.6.2. Implementation considerations: treatment of TB meningitis
▶
▶
▶
▶
-
5.2.7. Recommended dosing of first-line medicines in children
- 5.2.7.1. Recommended dosages for first-line TB medicines
- 5.2.7.2. Dosage tables and formulations for treatment of drug-susceptible TB in children and adolescents
- 5.2.7.3. Dosing table for the short intensive TB meningitis regimen
- 5.2.7.4. Dosing of first-line medicines in older children and adolescents over 25 kg (excluding the short intensive TB meningitis regimen)
- 5.2.7.5. Pyridoxine supplementation
▶
▶
▶
▶
-
5.2.8. Additional management considerations
▶
▶
▶
▶
- 5.2.9. Nutritional support
-
5.2.10. Management of adverse events from medicines used to treat drug-susceptible TB
- 5.2.10.1. Hepatotoxicity
- 5.2.10.2. Peripheral neuropathy
- 5.2.10.3. Optic neuritis
- 5.2.10.4. Overview of common adverse events and their management
▶
▶
▶
▶
- 5.2.11. Treatment adherence
-
5.2.12. Follow-up and monitoring of children and adolescents on TB treatment
- 5.2.12.1. Follow-up after treatment completion
- 5.2.12.2. Treatment interruption
- 5.2.12.3. Treatment failure
- 5.2.12.4. Children and adolescents requiring retreatment for TB
- 5.2.12.5. Registration of TB treatment in children and adolescents
▶
▶
▶
▶
▶
▶
▶
▶
- Key messages: treatment of DS-TB
-
5.3 Treatment of multidrug-resistant and rifampicin-resistant TB in children and adolescents
- 5.3.1. Identifying children who should be treated for multidrug-resistant and rifampicin-resistant TB
-
5.3.2. Multidrug-resistant and rifampicin-resistant TB treatment regimens for children and adolescents
- 5.3.2.1. Overview and approach to selecting a treatment regimen
- 5.3.2.2. Shorter all-oral bedaquiline-containing regimen for multidrug-resistant and rifampicin-resistant TB in children
- 5.3.2.3. Longer individualized regimens for children with multidrug-resistant and rifampicin-resistant TB who are not eligible for the standardized all-oral bedaquiline-containing regimen
- 5.3.2.4. Practical approach to designing individualized multidrug-resistant and rifampicin-resistant TB treatment regimens
- 5.3.2.5. Special considerations: TB meningitis
- 5.3.2.6. Special considerations: TB/HIV coinfection
▶
▶
▶
▶
-
5.3.3. Dosing and formulations of second-line TB medicines in children and young adolescents
▶
▶
▶
▶
-
5.3.4. Monitoring of children and adolescents on multidrugresistant and rifampicin-resistant TB treatment
▶
▶
▶
▶
▶
▶
▶
▶
- Key messages: treatment of DR-TB
-
5.4 Practical guidance for assessment and management of post-TB health in children and adolescents
- 5.4.1. Post-TB health
- 5.4.2. Post-TB meningitis in children and adolescents
- 5.4.3. Post-TB lung disease in children and adolescents
- 5.4.4. Post-TB osteoarticular disease in children and adolescents
- 5.4.5. Post-TB health-related quality of life
▶
▶
▶
▶
▶
▶
▶
▶
-
6. Models of TB care for children and adolescents
- 6.1 Introduction
-
6.2 Decentralized, family-centred, integrated TB services
-
6.2.1. Implementation considerations
- 6.2.1.1. Stakeholder engagement
- 6.2.1.2. Regulatory framework and policy guidance
- 6.2.1.3. Health workforce
- 6.2.1.4. Treatment support
- 6.2.1.5. Recording and reporting
- 6.2.1.6. Access to diagnostic supplies and child-friendly formulations of TB medicines
- 6.2.1.7. Resource requirements
- 6.2.1.8. Opportunities for integration of TB services into other services
- 6.2.1.9. Socioeconomic impact of TB on children, adolescents and families
- 6.2.1.10. Examples of country experiences in development of family-centred, decentralized and integrated TB services for children and adolescents
▶
▶
▶
▶
▶
▶
▶
▶
-
6.2.1. Implementation considerations
-
6.3 Private-sector involvement in care for children and adolescents with TB
- 6.3.1. Background
- 6.3.2. Rationale
- 6.3.3. Benefits of involving the private sector in TB care
- 6.3.4. Implementation considerations
▶
▶
▶
▶
-
6.4 Differentiated TB service delivery
▶
▶
▶
▶
- 6.5 TB and health emergencies
- Key messages
▶
▶
▶
▶
-
7. Special situations
-
7.1 Management of TB in children and adolescents living with HIV
- 7.1.1. Introduction
- 7.1.2. TB screening in children and adolescents living with HIV
- 7.1.3. Prevention of TB in children and adolescents living with HIV
- 7.1.4. Diagnosis of TB in children and adolescents living with HIV
- 7.1.5. Treatment of TB in children and adolescents living with HIV
- 7.1.6. Co-trimoxazole preventive therapy
-
7.1.7. Antiretroviral therapy
- 7.1.7.1. Timing of antiretroviral therapy
- 7.1.7.2. Choice of antiretroviral therapy regimen
- 7.1.7.3. Adjustments to antiretroviral therapy regimens with TB treatment
- 7.1.7.4. TPT in children and adolescents living with HIV on antiretroviral therapy
▶
▶
▶
▶
- 7.1.8. Immune reconstitution inflammatory syndrome
▶
▶
▶
▶
-
7.2 TB in pregnancy and management of newborns of mothers with TB disease
- 7.2.1. Screening for TB in pregnant women living with HIV
-
7.2.2. Congenital and neonatal TB
▶
▶
▶
▶
- 7.2.3. Management of asymptomatic neonates of mothers with TB
▶
▶
▶
▶
-
7.3 Palliative care for children and adolescents with TB
- 7.3.1. Introduction
- 7.3.2. Palliative care for people with TB
- 7.3.3. Palliative care for children and adolescents with TB
▶
▶
▶
▶
-
7.4 Care for adolescents with or at risk of TB
- 7.4.1. Physical and mental health
- 7.4.2. Connectedness and positive contribution to society
- 7.4.3. Safety and a supportive environment
- 7.4.4. Learning, competence, education, skills and employability
- 7.4.5. Agency and resilience
- 7.4.6. Substance abuse and late presentation to care
- 7.4.7. Poor adherence
- 7.4.8. Making TB services more adolescent-friendly
▶
▶
▶
▶
- 7.5 TB in children with severe acute pneumonia
-
7.6 Management of children with TB and malnutrition
- 7.6.1. Introduction
- 7.6.2. Diagnosis and treatment of TB in children with malnutrition
- 7.6.3. Nutritional care for children and adolescents with TB
▶
▶
▶
▶
- Key messages
▶
▶
▶
▶
-
7.1 Management of TB in children and adolescents living with HIV
- 8. References
- Annex 1. Selected resources on child and adolescent TB
- Annex 2. Tuberculin skin testing: administration, reading and interpretation
- Annex 3. Sample collection methods
- Annex 4. Standard operating procedures for sample collection methods
- Annex 5. Treatment decision algorithms
- Annex 6. Dosing of medicines used in second-line multidrug-resistant TB regimens by weight band (below 46 kg)
- Annex 7. Overview of options for neurocognitive and functional testing at end of treatment for TB meningitis
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 5: Management of tuberculosis in children and adolescents
-
Module 6: Comorbidities
-
Module 6: Tuberculosis and comorbidities
- Introduction
-
Mental health conditions and substance use disorders
- Acknowledgements
- Abbreviations
- Definitions
- 1. Mental health conditions and substance use disorders: background and rationale
- 2. People-centred care for mental health conditions and substance use disorders in people affected by TB
-
3. Identifying and managing care for mental health conditions and substance use disorders in people affected by TB
▶
▶
▶
▶
-
4. Special considerations
▶
▶
▶
▶
- References
- Annex. WHO resources for mental and substance use disorders
▶
▶
▶
▶
-
HIV
- Acknowledgements
- Abbreviations and acronyms
- Definitions
-
1. Background and rationale
- 1.1 Background and burden of HIV-associated TB
- 1.2 Development of the document
- 1.3 Summary of recommendations
▶
▶
▶
▶
-
2. Establish and strengthen collaboration across health programmes and across sectors for delivering people-centred services for HIV-associated TB
- 2.1 Strengthen governance and accountability for TB/HIV collaborative activities
- 2.2 Conduct an analysis of access to quality services for TB and HIV
- 2.3 Coordinate planning and resource mobilization for collaborative action
- 2.4 Implement and scale up people-centred services for HIV-associated TB
- 2.5 Strengthen monitoring, evaluation and research
▶
▶
▶
▶
-
3. Reduce the burden of TB among people living with HIV
- 3.1 TB screening
- 3.2 TB diagnosis
- 3.3 Algorithms for informing the decision to treat TB infection or TB disease among people living with HIV
- 3.4 TB treatment
- 3.5 Prevention of TB
▶
▶
▶
▶
-
4. Reduce the burden of HIV among people with TB
- 4.1 HIV testing services for people with presumptive and diagnosed TB
- 4.2 HIV treatment and care for people living with HIV diagnosed with TB
- 4.3 HIV prevention
▶
▶
▶
▶
- References
- Annex 1. Monitoring and evaluation of collaborative TB/HIV activities
- Annex 2. Methods for algorithms for diagnosis of TB in people living with HIV
- Annex 3. Checklist for TB infection prevention and control
- Annex 4. Package of care for advanced HIV disease
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
▶
-
Module 6: Tuberculosis and comorbidities
▶
▶
-
Module 1: Prevention
- Training Catalogue
- TB Drug Dosage Finder
 Feedback
Feedback