Book traversal links for 6.1 Drug safety and adverse drug reactions
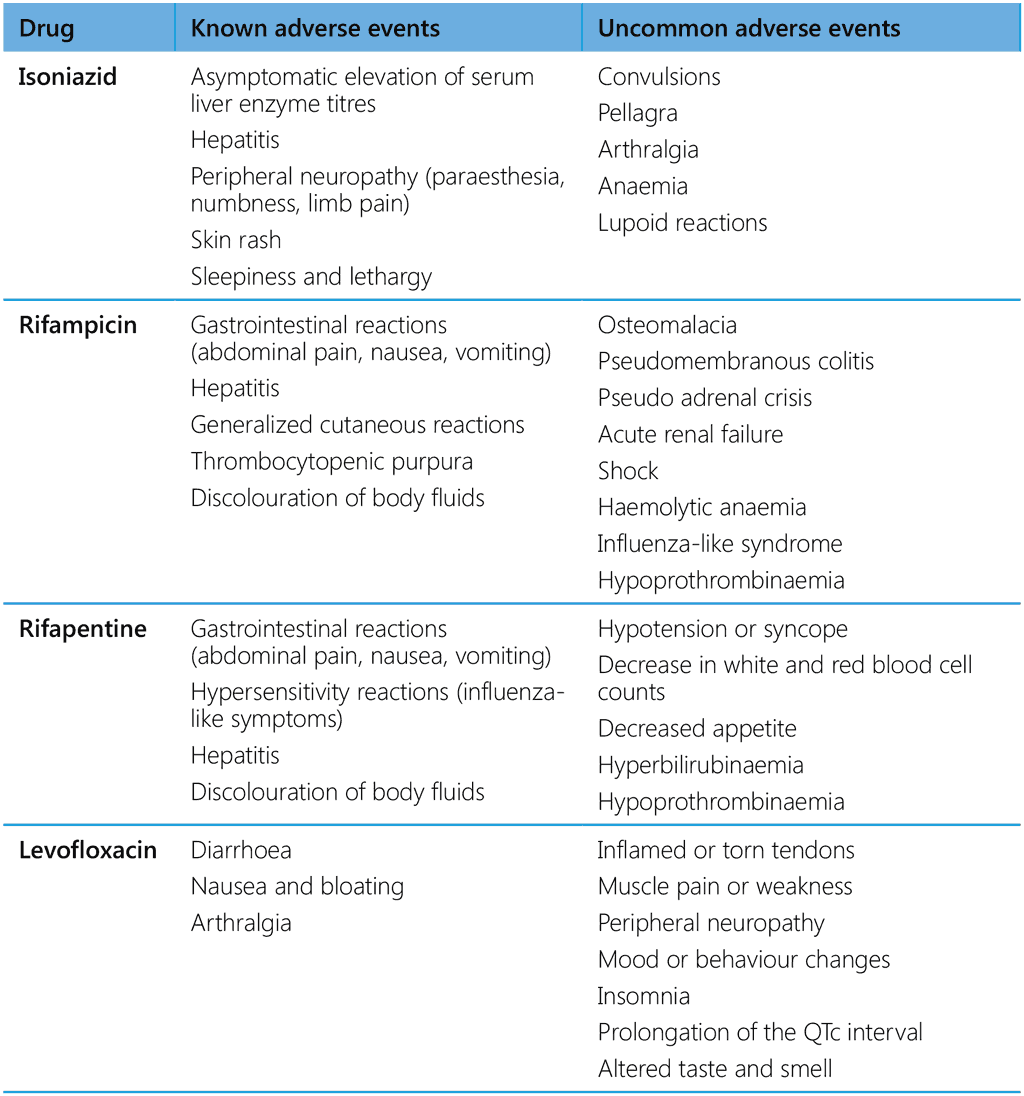
Overall, serious adverse events leading to death or requiring withdrawal of TPT occur rarely. It is nevertheless critical to identify any sign of drug toxicity as soon as possible and to manage it immediately, particularly because people on TPT are usually healthy. Unmanaged drug toxicity may not only harm individuals but can also damage the reputation of a programme and result in widescale suspension of TPT due to loss of public confidence. As in any preventive action, health-care providers must weigh the risks and benefits of TPT for each individual. Obtaining a detailed, accurate medical history (including medicines being taken and past adverse drug reactions) and keeping up-to-date information at every contact with a person on TPT can help to identify people who should be closely monitored and the most appropriate course of action if an adverse event occurs. Individuals on TPT should also be monitored at regular scheduled visits (monthly if feasible or as required for individual care or national programmes). Table 5 summarizes adverse events associated with currently used TPT drugs.
Table 5. Documented adverse events with drugs used for TPT
In a network meta-analysis conducted in 2014 (updated in 2017), adverse events associated with the use of standard isoniazid regimen were compared with those associated with 3 or 4R and 3 or 4HR (55,129). The regimen with rifampicin only and that with rifampicin plus isoniazid were reported to be associated with a lower risk for hepatotoxicity than isoniazid monotherapy. Another systematic review, which included data from 23 randomized and 55 nonrandomized studies, reported high hepatotoxicity rates with 6H or 9H (2–6%) and the lowest rates with 3HP (1%) and 3R or 4R (0.01–2%) (130). The review, however, clearly stated the overall weak documentation of adverse events, heterogeneity in the data (different definitions of hepatotoxicity) and a high risk of bias in the studies. The data do provide indications of the frequency of adverse events and events that eventually require stopping preventive treatment. The highest median rates of withdrawal due to adverse events were associated with 6H, followed by 9H, and the lowest rates with 3HP. Possible hypersensitivity reactions were reported in up to 4% of individuals on 3HP and 2% on 3HR. Few deaths due to any cause during TPT have been reported. In the studies included in the analysis, no deaths were reported among participants on 9H, 3HP or 3–4R, while a few deaths occurred in those on 6H and 3–4HR, largely among people with HIV who were not on ART and people with other comorbid conditions. Reassuringly, anaphylaxis was rarely reported with any regimen.
A systematic review and meta-analysis published in 2023 estimated the cumulative incidence of all types of adverse events and of hepatotoxicity associated with TPT regimens containing isoniazid and/or rifamycins (131). Children had a very low incidence of adverse events with all TPT regimens, including hepatotoxic adverse events of any severity and grade 3 and 4 severity or that resulted in drug discontinuation. Studies in which more than 50% of people had HIV had lower rates of adverse events of any type and severity and lower rates of adverse events leading to drug discontinuation. The incidence of adverse events leading to TPT discontinuation in pregnancy was 0.8% (95% CI 0.2% ; 3.3%). One death was related to TPT with rifamycin-based regimens and several related to IPT regimens, but no TPT-related deaths were identified in the studies in children. Pooled analysis of the studies in this review show a rate of any adverse events related to the study drug of about 7% (95% CI 5.3% ; 9.3%), with 16.4% (8.7% ; 28.7%) grades 1 and 2 and 2.4% (1.7% ; 3.5%) grades 3 and 4. The cumulative incidence of TPT discontinuation due to adverse events was 3.7% (95% CI 3.1% ; 4.5%). Adverse events leading to permanent drug discontinuation among children was < 1%. Table 6 shows the frequency of grades 3 and 4 events and discontinuation of TPT due to adverse events in recent studies and from two unpublished studies of Lfx use as TPT for MDR-TB (see Annex 5 of the second edition of WHO TPT guidelines, 2024) (62,120,131). The frequency of drug discontinuation due to adverse events varied from 0.6% with 3HP to 3.8% with the 3HR regimen. Several studies in highburden countries (132,133) align with the discontinuation rate reported in the systematic review of 1.7% (0.5% ; 4.9%) and for 4R and 1HP of < 3% (134).
Table 6. Grade 3–4 adverse events and discontinuation of treatment due to adverse events in people on TPT
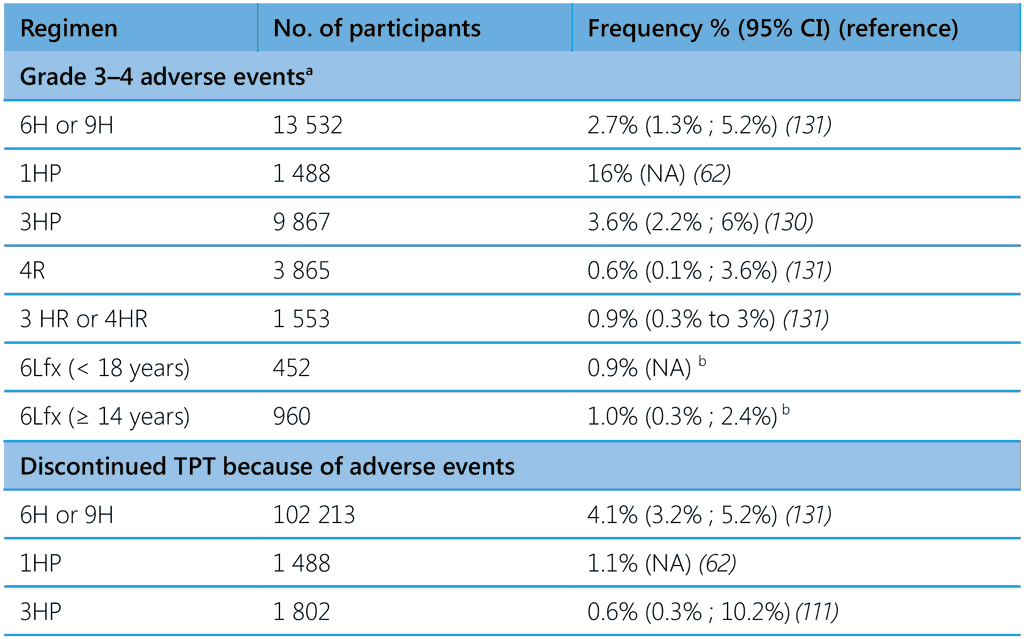
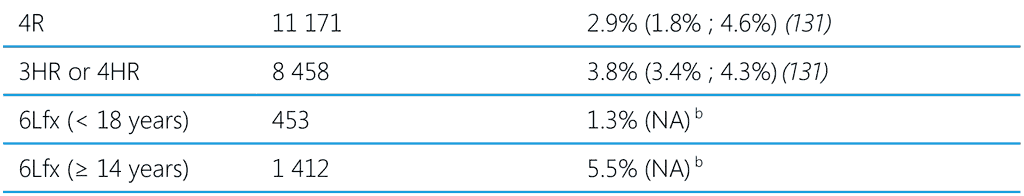
1HP, 1 month of daily rifapentine plus isoniazid; 3HP, 3 months of weekly rifapentine plus isoniazid; 3HR or 4HR, 3 or 4 months of daily rifampicin plus isoniazid; 4R, 4 months of daily rifampicin monotherapy; 6H or 9H, 6 or 9 months of daily isoniazid monotherapy; 6Lfx, 6 months of daily levofloxacin monotherapy; NA, not available; TPT, TB preventive treatment.
a Grade-3 adverse event: medically significant but not an imminently life-threatening; Grade-4 adverse event: life-threatening
b Unpublished data from TB CHAMP and V-QUIN trials (see Annex 5 of (13))
The following sections provide further details on of the adverse events associated with specific drugs.
6.1.1 Isoniazid
- Asymptomatic elevation of serum liver enzyme concentrations occurs in 10–20% of people taking isoniazid, which usually return to normal even if treatment is continued.
- Clinical hepatitis leading to death occurs in about 0.1% of people taking isoniazid and is more common when the drug is combined with other hepatotoxic agents. Factors that may increase either the rate or severity of hepatitis include daily alcohol consumption, underlying liver disease or risk for liver disease, age > 65 years, and concurrent use of other medications that are metabolized in the liver. Symptomatic hepatitis is rare among people < 20 years, although severe and fatal cases have been reported.
- Peripheral neuropathy (paraesthesia, numbness and limb pain) occurs in < 0.2% of people taking isoniazid at normal doses. It is more likely in the presence of other conditions associated with neuropathy, such as diabetes, malnutrition, HIV, renal failure and problem alcohol use.
- Isoniazid is recognized as a secondary cause of pellagra, as it interrupts cellular niacin (vitamin B3) production in people with underlying nutritional deficiency. Niacin plays a vital role in numerous metabolic processes. Pellagra is clinically diagnosed by its characteristic skin rash; other symptoms include diarrhoea and neuropsychiatric changes. Populations at increased risk for pellagra include people who consume alcohol in excess and those with an undiversified reliance on unfortified maize staples.
- Other known adverse drug reactions due to isoniazid are skin rash, sleepiness and lethargy.
6.1.2 Rifampicin
- Gastrointestinal symptoms, such as nausea, anorexia and abdominal pain, are rarely severe enough to discontinue treatment.
- Hepatotoxicity, evidenced by transient asymptomatic hyperbilirubinaemia, occurs in 0.6% of people taking rifampicin. Hepatitis is more likely when rifampicin is combined with isoniazid.
- Cutaneous reactions, such as itching (pruritus), with or without a rash, may occur in about 6% of people taking rifampicin. They are generally self-limiting and may not be true hypersensitivity reactions. Continuation of treatment may be possible.
- Rifamycin hypersensitivity syndrome has been reported with use of rifampicin, characterized by influenza-like symptoms. The syndrome, when it occurs, typically develops a few weeks after the start, and, in most instances, TPT can be tolerated subsequently (132,133). Rarely, rifamycins are associated with hypersensitivity reactions, including hypotension, nephritis or thrombocytopenia manifested by symptoms such as fever, headache, dizziness or light-headedness, musculoskeletal pain, petechiae and pruritus.
- Orange discolouration of body fluids is expected and is harmless.
6.1.3 Rifapentine
- Overall, rifapentine in TPT is associated with fewer adverse events and is well tolerated, even by people with various degrees of hepatic dysfunction (134).
- Clinically significant systemic drug reactions, most of which are influenza-like, are reported in up to 3.5% individuals receiving 3HP, most cases being mild and resolving within 24 h (135). Clinical monitoring and continued vigilance for systemic drug reactions are nevertheless warranted in PMTPT. While there have been reports of hypotension or syncope after taking 3HP, hypersensitivity is uncommon.
- Other common adverse reactions include a change in the colour of body fluids to orange–red (benign), gastrointestinal side-effects (such as nausea, vomiting, loss of appetite), decreased white and red blood cell counts, skin rash or itching, joint pain and red eyes (136).
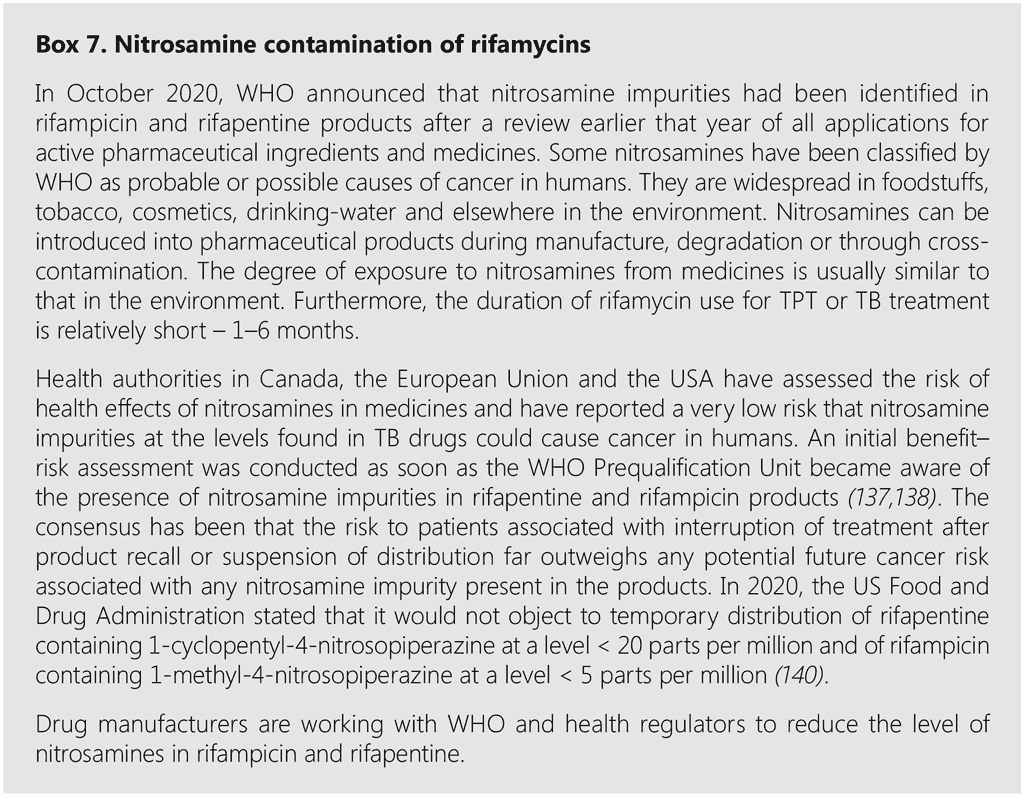
Recently, concern has been raised about reports of nitrosamine impurities in rifamycins. Box 7 summarizes the context and measures being implemented to mitigate any potential risk.
6.1.4 Levofloxacin
Results from the TB CHAMP and V-QUIN studies were reviewed by the WHO GDG that developed the second edition of the TPT guidelines in 2024. These showed an important difference in the risks for adverse events between children and adults, with very good tolerance in children but less tolerability with increasing age. One or more adverse events of any grade was reported in about 32% of adolescents and adults taking Lfx in the V-QUIN trial, most being grade 1 or 2. Serious adverse events were infrequent, about 1% of participants developing grade 3 or 4 events, the occurrence of which was not statistically significantly different from that in the placebo arm (Table 6). Pooled analysis of data from the two trials shows that Lfx was associated with more musculoskeletal events (arthritis, arthralgia or tendonitis) in adolescents and adults, mostly grade 1 or 2. Treatment discontinuation for adverse events, although uncommon, occurred more frequently among adolescents and adults than among children, which may have implications for adherence by adolescents and adults.
A systematic review conducted to inform the 2022 update of the consolidated guidelines on treatment of DR-TB showed that Lfx is generally safe, with some mild or moderate drug-related adverse events in children but no grade 3 or 4 or serious adverse events (141). Recent alerts highlight the safety concerns associated with prolonged use of fluoroquinolones in humans (142–144). The most commonly reported adverse events are dizziness, headache, nausea and abdominal pain (145). Although the adverse events may be mild, they might still require discontinuation of therapy. Fluoroquinolones increase the risk of tendon injuries, and older age and concomitant use of corticosteroids may be additional risk factors for tendinopathy (146).
6.2 Management of adverse events
As individuals who receive TPT are otherwise healthy, adverse events during TPT must be minimized. Most adverse events in people on TPT are mild and self-limiting. Conservative management and continuation under observation can be considered by the health-care provider if there are mild-tomoderate adverse events; however, if a severe adverse reaction occurs, TPT must be discontinued immediately and supportive medical care provided.
As a part of initial counselling, health workers should stress the importance and rationale for TPT and of completing the course and re-emphasize the risk associated with TB disease. A person on TPT should also be told about likely adverse events and be urged to contact their health-care provider if they develop events between visits that suggest drug toxicity (such as loss of appetite, persistent fatigue or weakness, abdominal discomfort, nausea, vomiting, dark-coloured urine, pale stools, rash or itching, yellow skin or eyes, tingling or numbness in the hands or feet). If a health worker cannot be consulted at the onset of such symptoms, the person on TPT should immediately stop treatment. People treated with rifamycins should be warned about pink discolouration of secretions due to this medicine, and people on Lfx should be informed of the rare occurrence of musculoskeletal symptoms such as tendonitis.
Clinician discretion should be exercised, and a complete history, including concomitant medications and supplements, must be taken. The following steps may help in assessing management of adverse events.
- How severe is the adverse event (mild, moderate, severe)?
- How serious is the event (likely to lead to death or life-threatening; hospitalization or prolongation of hospitalization required; persistent significant disability; congenital anomaly)?
- What should immediate management consist of (reassurance, symptomatic relief, discontinuation of TPT or requires an intervention to avert a severe outcome)?
- What is the underlying cause (the drug, other factors)?
- How will the adverse event affect future adherence (tolerability, consideration of substitution with an alternative regimen)?
- What is the next step (continue or restart, substitute, follow up and reassess, definitive halt)?
Once a person is started on TPT, routine monitoring should include evaluation of tolerability and adherence. The following should be checked at every contact:
- signs or symptoms of TB disease (“breakthrough” disease or missed diagnosis at start of TPT). If the person starts a cough, unexplained weight loss, fever or night sweats, they should immediately let the provider know and undergo testing. In younger children other, non-specific features such as failure to thrive, lack of playfulness and reduced appetite should be carefully monitored as they may be early signs to TB disease.
- pregnancy: continue TPT; if on 1HP or 3HP monitor closely or consider switching to an alternative TPT regimen such as 3HR;
- adverse event: type, onset and duration, severity, seriousness;
- assessment of adherence and provision of necessary support: any interruption to treatment should be discussed with the person on treatment and her or his treatment supporter, and interventions instituted to address problems in adherence;
- occurrence of another disease, such as malaria or diabetes;
- relevant physical examination;
- any medication (including traditional cures) that may interact with TPT; and
- liver function tests for individuals who had raised levels at baseline or at a previous visit, or with a history of regular use of alcohol. (See also section 5.)
National programmes should establish a mechanism for systematic recording and management of any adverse event reported by people on TPT. In addition to prompt management, suspected or confirmed adverse drug reactions should be reported to the national authority responsible for pharmacovigilance as per local regulations. Patient files should be reviewed regularly to assess the most frequent types of adverse events and programme implementation adjusted to minimize them.
The following section provides guidance on management of the most common adverse drug reactions related to TPT drugs and regimens.
6.2.1 Isoniazid and rifampicin
Drug-induced hepatitis
- Features that indicate stopping medication: Transient, asymptomatic increases in serum liver transaminases during the early weeks of treatment. Treatment need not be interrupted or changed unless there is anorexia, malaise, vomiting or clinically evident jaundice. Clinical features of concern include protracted vomiting, mental changes and signs of bleeding, all of which suggest impending acute liver failure and require immediate discontinuation of medication.
- Management of jaundice and other severe features: If jaundice or any of the clinical features that suggest acute liver failure develop, all drugs must be stopped until jaundice or hepatic symptoms have resolved and liver enzymes have returned to baseline levels. If liver enzymes cannot be measured, it is advisable to wait 2 weeks after jaundice has disappeared before starting TPT. Other causes of hepatitis should be explored. Younger people with underlying risk factors for liver disease should be monitored clinically with the same precautions as older people.
- Reintroduction: Once hepatitis has resolved, the same drug regimen can be reintroduced, either gradually or all at once (“rechallenge”). If hepatitis was life-threatening and was unlikely to have been caused by something else (such as alcohol or viral infection), it is safer to switch to an alternative regimen.
Skin reactions
- Itching with no or a mild rash: Symptomatic treatment with antihistamines may be tried and TPT continued.
- Itching with moderate or severe rash: If the rash is severe or if there is evidence of mucosal involvement, hypotension or severe illness, corticosteroid treatment should be considered. Oral prednisolone (40–60 mg) should be given daily until there is a response; the dose should then be reduced gradually over the following days according to the clinical response. TPT should be withheld until the reaction has completely subsided. If the initial cutaneous reaction was severe, the full dose may be increased with smaller initial challenge doses. If a severe reaction occurs, the suspected medicine should not be given again, and an alternative regimen may be considered.
- People who develop isoniazid-associated pellagra should discontinue isoniazid and take high-dose nicotinamide (a form of vitamin B3). Full recovery is possible. Pellagra may result in severe illness or death if untreated (147). The recommended treatment for pellagra is 300 mg of nicotinamide daily for 3–4 weeks. Good dietary sources of vitamin B3 are similar to those of vitamin B6 (see section 5.2.2).
Peripheral neuropathy
- To prevent peripheral neuropathy, administer 10–25 mg of vitamin B6 (pyridoxine) daily
- For established peripheral neuropathy, give pyridoxine at a higher dose of 100–200 mg daily. (See section 5 for more details.)
- Routine pyridoxine supplementation is recommended only in such conditions or when there is another risk of isoniazid toxicity (e.g. individuals who are slow acetylators or in a setting with known high levels of slow acetylators).
Gastrointestinal reactions
- Abdominal pain, nausea or vomiting may be associated with rifampicin use. If the symptoms are mild, the episode is usually self-limiting, and reassurance may suffice. If gastrointestinal upset is severe enough to risk interruption of treatment, suspend rifampicin for three or four doses, use medications that provide symptomatic relief (such as metoclopramide to counteract nausea and vomiting), or, as a last resort, give rifampicin with small amounts of food to allow continued use of the medicine. Although concomitant ingestion of food reduces the absorption of rifampicin slightly, this is preferable to complete discontinuation of TPT.
Lethargy: reassurance.
Discolouration of body secretions: red or orange urine, tears, semen and sweat are normal in people taking rifamycins. They should be reassured that the condition is innocuous and reversible.
6.2.2 Isoniazid and rifapentine
Most adverse drug reactions associated with HP regimens are mild, self-resolving and without sequelae. The following specific actions may be considered during management of potential adverse drug reactions after treatment with 3HP and 1HP.
- Influenza-like syndrome (attacks of fever, chills and malaise, sometimes with headache, dizziness or bone pain)
- Influenza-like and other acute symptoms appear shortly after taking a dose of rifapentinecontaining TPT, most commonly with the third dose.
- If the symptoms are mild and not increasing, continue treatment and observe closely.
- If the symptoms are moderate to severe, consider alternative TPT options without rifamycin (such as 6H).
- Drug-associated fever
- Consider reintroduction of TPT if body temperature stays below 39 o C, but stop TPT permanently if fever recurs.
- If body temperature is > 39 oC after a previous episode of drug-associated fever, stop TPT, and do not reintroduce HP.
- Gastrointestinal reactions (persistent nausea, frequent vomiting and/or persistent episodes of unformed watery stools)
- Administer antiemetic or anti-diarrhoeal medication, and consider reintroducing HP with caution once the symptoms have resolved,
- If the nausea, vomiting or diarrhoea requires aggressive rehydration, stop TPT, and do not reintroduce HP.
- Cutaneous reactions
- In the case of diffuse rash (no vesicles) or diffuse rash with limited vesicles, stop, and consider reintroduction with caution.
- If there are extensive bullous lesions, ulceration of mucous membranes or Stevens Johnson or toxic epidermal necrolysis, contact a specialist and administer steroids.
- Other hypersensitivity reactions (hypotension, acute bronchospasm, conjunctivitis, thrombocytopenia)
- Assess the clinical severity of the symptoms, and, if they are severe, consider an alternative TPT option without rifamycin (6H).
- Hypersensitivity usually resolves quickly after medication is stopped, with no long-term consequences.
- Hepatitis
- Early signs of hepatotoxicity include weakness, fatigue, loss of appetite and persistent nausea. They should be identified early, as hepatotoxicity is reversible without permanent sequelae. Late signs of hepatotoxicity include liver tenderness, liver enlargement (hepatomegaly) and jaundice.
- Stop HP, and consider reintroduction with caution if alanine and aspartate aminotransferase levels are more than five times the upper limits of normal in the absence of symptoms.
- Stop and do not reintroduce HP if alanine and aspartate aminotransferase levels are five or more times the upper limit of normal in the absence of symptoms or three or more times the upper limit of normal in the presence of symptoms.
- Psychosis
- Undertake a psychiatric evaluation, consider antipsychotic therapy, and provide pyridoxine supplementation. Stop, and do not reintroduce isoniazid if the psychosis is attributable to isoniazid.
- Seizures
- Withhold isoniazid pending resolution of seizures, and evaluate the possible causes (107); stop and do not reintroduce isoniazid if the seizures are attributable to isoniazid.
Rifamycins are potent enzyme inducers and may induce drug–drug interactions. (See also section 6.3.)
6.2.3 Levofloxacin
Patients should be advised to inform their health-care provider immediately if any of the following occurs:
- pain, swelling or tearing of a tendon (such as the back of the ankle of elbow) or muscle or joint pain;
- severe diarrhoea (watery or bloody);
- paraesthesiae (tingling or burning sensations in the peripheries);
- seizures, epilepsy, change in mood or behaviour; or
- low blood sugar symptom (headache, hunger, sweating, irritability, dizziness, nausea, fast heart rate or feeling anxious or shaky).
The specific course of action will depend on the adverse event reported, such as use of pain medication and pyridoxine for neuropathy. It is important to note whether the adverse events are worsening, in which case, a doctor should be consulted. Sunscreen lotions should be used for Lfx-induced sun sensitivity. Some of the adverse events may take time to resolve, and the person should be counselled to exercise patience.
6.3 Drug–drug interactions
6.3.1 Rifamycins and ARV
When rifamycins and ARVs are given together, the effect of either drug on the body may be changed. A drug–drug interaction can increase or decrease the action of either or both drugs, reduce efficacy or cause adverse events. Rifamycins are potent inducers of metabolizing enzymes, including cytochrome P450 enzymes, and may therefore interfere with medicines that depend on this metabolic pathway, accelerating their elimination. Rifampicin in particular is a potent inducer of hepatic CYP 450 (mostly 3A and 2C sub-families), P-glycoprotein and uridine diphosphate glucuronosyltransferase 1A enzymes. Similarly, rifapentine induces P450 enzymes, specifically the CYP3A4, CYP2C8 and CYP2C9 isoenzymes (148). Rifampicin and rifapentine are similarly potent inducers, while rifabutin is less powerful.
In general, care should be taken when prescribing regimens containing rifampicin and rifapentine to people with HIV who are on ART. Rifamycins accelerate the metabolism of some ARV drugs; thus, co-administration of these ARVs with rifamycins may cause HIV treatment failure or resistance. The ARVs most affected by CYP 450 induction due to rifamycin include all PIs, non-nucleoside reverse transcriptase inhibitor (NNRTIs), integrase strand transfer inhibitors (such as dolutegravir) and CCR5 antagonists (such as maraviroc). These regimens can significantly decrease the concentrations of boosted protease inhibitors or nevirapine and should not be co-administered, including to HIVexposed infants on TPT. While dose adjustment is not required when rifampicin is co-administered with efavirenz, the dose of dolutegravir should be increased to 50 mg twice daily for adults when given with rifampicin (149). This dose is well tolerated and is of equivalent efficacy to efavirenz in viral suppression and recovery of CD4 cell count (150). 3HP can be administered to people receiving efavirenz-based ARV regimens without dose adjustment (151). Administration of rifapentine with raltegravir is also safe and well tolerated (152).
Rifamycins also interact with many other medicines (see Table 6). Sound clinical judgement is therefore required when these medicines are to be co-administered with rifamycin-based TPT, either by avoiding them or adjusting their dose (153).
6.3.2 Co-administration of rifapentine and dolutegravir
While once-weekly rifapentine is known to reduce exposure to dolutegravir, the blood levels of dolutegravir remain above the target concentrations for viral suppression in adults taking both medicines. One study showed that a reduction in dolutegravir concentration – even by 75–80% – is unlikely to be clinically significant, as even a dose of 10 mg dolutegravir once daily (with nucleotide reverse transcriptase inhibitor backbone) results in high rates of virological suppression over 96 weeks, similar to those with an efavirenz-containing regimen (154). Dolutegravir can therefore be given with weekly rifapentine without modifying the dose.
The results of a phase 1/2 clinical trial of 3HP and dolutegravir in adults with HIV showed good tolerance and viral load suppression, no adverse events related to 3HP higher than grade 3 and no reduction of dolutegravir levels sufficient to require dose adjustment (155). Recent work continues to support this position (156,157). Preliminary evidence from a phase 1/2 trial (DOLPHIN TOO) (158) also supports the immediate start of TPT among ART-naïve people starting a dolutegravir based regimen. When 3HP was administered to 50 people with HIV who were ART-naive and were started on dolutegravir-containing ART, high rates of viral suppression, comparable to those with 6H, were achieved, and no difference in Grade 3 or 4 adverse events was observed (151). Administration of rifapentine with raltegravir was also found to be safe and well tolerated (152). The 3HP regimen can be administered to patients receiving efavirenz-based antiretroviral regimens without dose adjustment, according to a study of their pharmacokinetics (153).
No dose adjustment is required when rifampicin is co-administered with efavirenz, and the two drugs can be used together safely. The dose of dolutegravir, however, should be increased to 50 mg twice daily when given with rifampicin. This dose is usually well tolerated and is of equivalent efficacy to efavirenz in viral suppression and recovery of the CD4 cell count.
More studies should be conducted on the pharmacokinetics of concomitant administration of 3HP with other medicines, particularly boosted PIs and tenofovir alafenamide, and should include both pregnant women and children. Studies of the doses of dolutegravir with daily rifapentine and whether the dose of dolutegravir should be adjusted with 1HP for adults and children are under way (ACTG 5372).
6.3.3 ARV options for concomitant administration with rifamycin-based TPT
ART should be changed to accommodate a certain TPT regimen with the utmost caution. The clinician should seriously weigh the risks versus the benefit of such a change, as frequent changes of ART are associated with loss of virological control and should hence be avoided to the extent possible, particularly when the person is virologically suppressed by current ART. In addition, changing to efavirenz-based ART in areas with high rates of NNRTI resistance (including many areas in subSaharan Africa) is not ideal. Overall, successful ART should have primacy in a decision over choice of TPT regimen. If changing the ART regimen is being considered for compatibility with rifamycincontaining TPT the following should be considered.
- Most nucleotide reverse transcriptase inhibitors and fusion inhibitors do not interact significantly with rifamycins.
- Pharmacokinetics data show no significant drug–drug interactions between rifapentine and the NNRTI efavirenz (159–161) or the integrase strand transfer inhibitor raltegravir (152).
- No significant drug–drug interactions have been reported with use of rifapentine and an ART regimen containing abacavir, emtricitabine, TDF, lamivudine or zidovudine. Regimens with efavirenz or raltegravir used in combination with either abacavir/lamivudine or TDF/emtricitabine can be used with 3HP.
Tenofovir alafenamide is a notable exception, as it is a P-glycoprotein substrate and may result in unacceptably low exposure to a rifamycin such as rifapentine. Concomitant administration of tenofovir alafenamide and a rifapentine should therefore be avoided until further data are available to support their concurrent use (162). Of note, tenofovir alafenamide given with rifampicin results in intracellular levels of the active drug tenofovir diphosphate similar to those with TDF alone, suggesting that this combination could be used; however, clinical data are limited (163).
6.3.4 Isoniazid
Isoniazid is known to inhibit certain cytochrome P-450 enzymes. Therefore, co-administration of isoniazid with drugs that undergo biotransformation through these metabolic pathways may decrease their elimination, thereby increasing exposure. Consequently, dosages of drugs metabolized by these enzymes might have to be adjusted when starting or stopping to maintain optimal therapeutic blood levels. Isoniazid has been reported to inhibit the metabolism of efavirenz, anticonvulsants, benzodiazepines, haloperidol, ketoconazole, theophylline and warfarin. The impact of the competing effects of rifampicin and isoniazid on the metabolism of these drugs is unknown, but the inducing effects of rifampicin tend to be more prominent (Table 7).
Table 7. Common drug–drug interactions of isoniazid and rifamycins
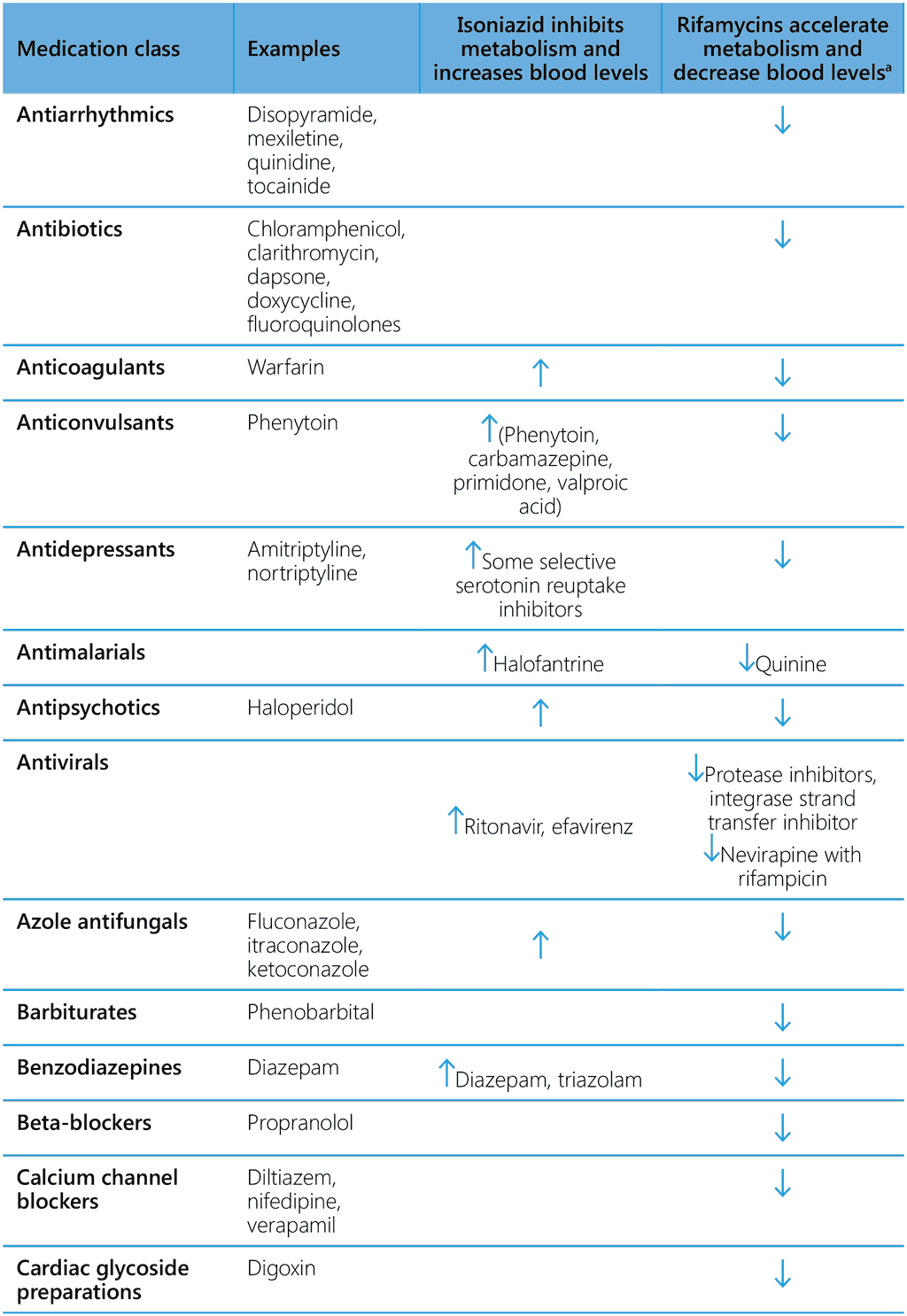
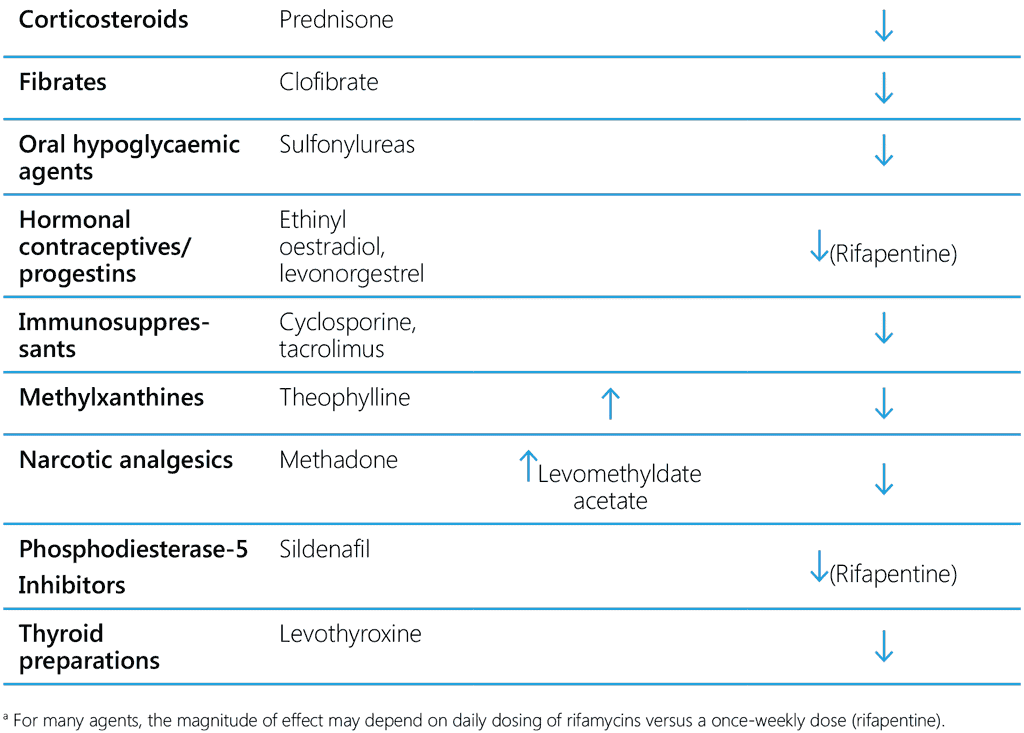
6.3.5 Rifamycin-based TPT and antimalaria treatment
As rifampicin and other rifamycins are potent CYP3A4 inducers, they decrease exposure to quinine in adults on malaria treatment, leading to a five-times increase in the rate of recrudescence (78). Similarly, concomitant administration with mefloquine reduces exposure to mefloquine by three times. A similar decrease in exposure was reported with co-administration of rifampicin and artemether, dihydroartemisinin and lumefantrine (decreases of nine, six and three times, respectively). There is insufficient evidence to change the current recommendations for dosing by body weight of these antimalarial agents, and close monitoring for recrudescence is advised. The following guidance may be applied until clear evidence becomes available on ways to increase exposure to antimalarial drugs.
- If a person has diagnosed malaria but is not yet on rifamycin-containing TPT, the episode of malaria should be prioritized and treated first.
- If a person has diagnosed malaria while on rifamycin-based TPT, malaria treatment should be started concomitantly and monitored clinically according to national guidelines to ensure that the malaria is cured. There is insufficient evidence to indicate that the doses of either TPT or artemisininbased combination therapy should be adjusted.
- If malaria recurs in a person on TPT, they should be retreated for malaria according to national guidelines. Preventive treatment should be withheld only if the new malaria treatment also includes drugs that are known to interact with rifamycins. TPT may be resumed once the episode of malaria is resolved.
- If a person meets the diagnostic criteria for severe malaria (impaired consciousness, low blood glucose, high bilirubin, jaundice, bleeding, anaemia, kidney failure and parasitaemia > 10%), TPT should be withheld, and the person treated urgently according to national guidelines. TPT should be recommenced only when the episode of malaria is fully resolved.
6.3.6 Levofloxacin
The absorption of Lfx is not influenced by food. There are no major interactions between milk or dairy products and third-generation fluoroquinolones. Concomitant steroid use may increase the risk of tendon rupture. Antacids (especially those containing aluminium), mineral supplements (e.g. iron or magnesium) or multivitamins may decrease absorption and should therefore be taken more than 2 h before or after this medication. The effect of warfarin may be enhanced by Lfx. Prothrombin time and international normalized ratio should be monitored, and the patient should be informed about the risk of bleeding. As Lfx may affect glucose metabolism, blood sugar levels should be monitored.
 Feedback
Feedback