Book traversal links for 5.3.4.2. Monitoring for adverse effects
Routine safety monitoring of treatment should generally follow the recommended approach in adults and should be guided by the known adverse effect profile of the medicines included in the regimen.
Table 5.14 summarizes the most common adverse effects of the medicines used for MDR/RR-TB.
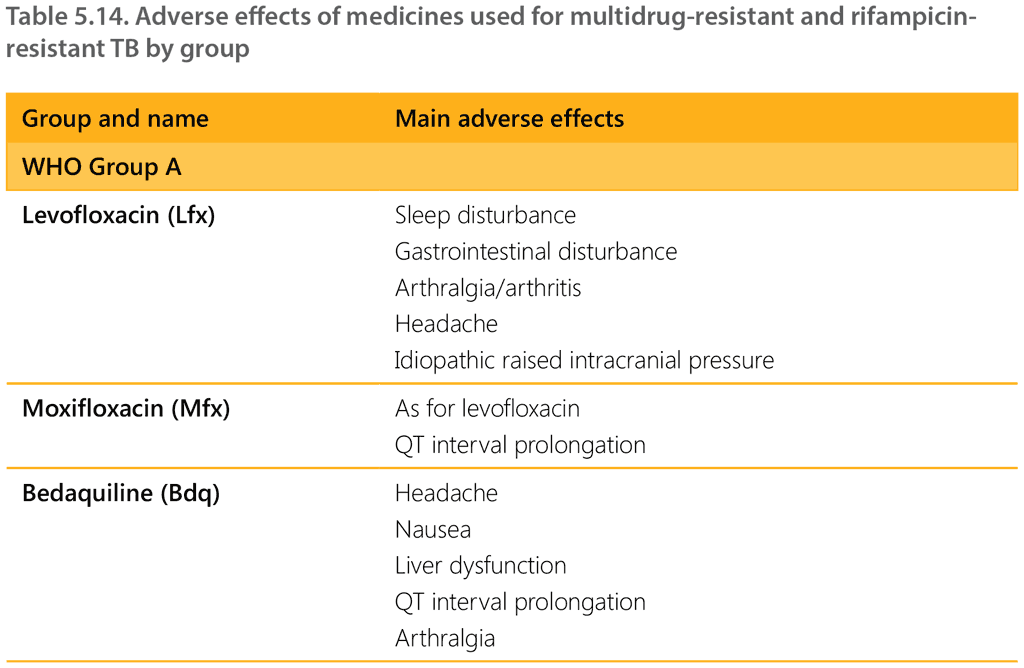
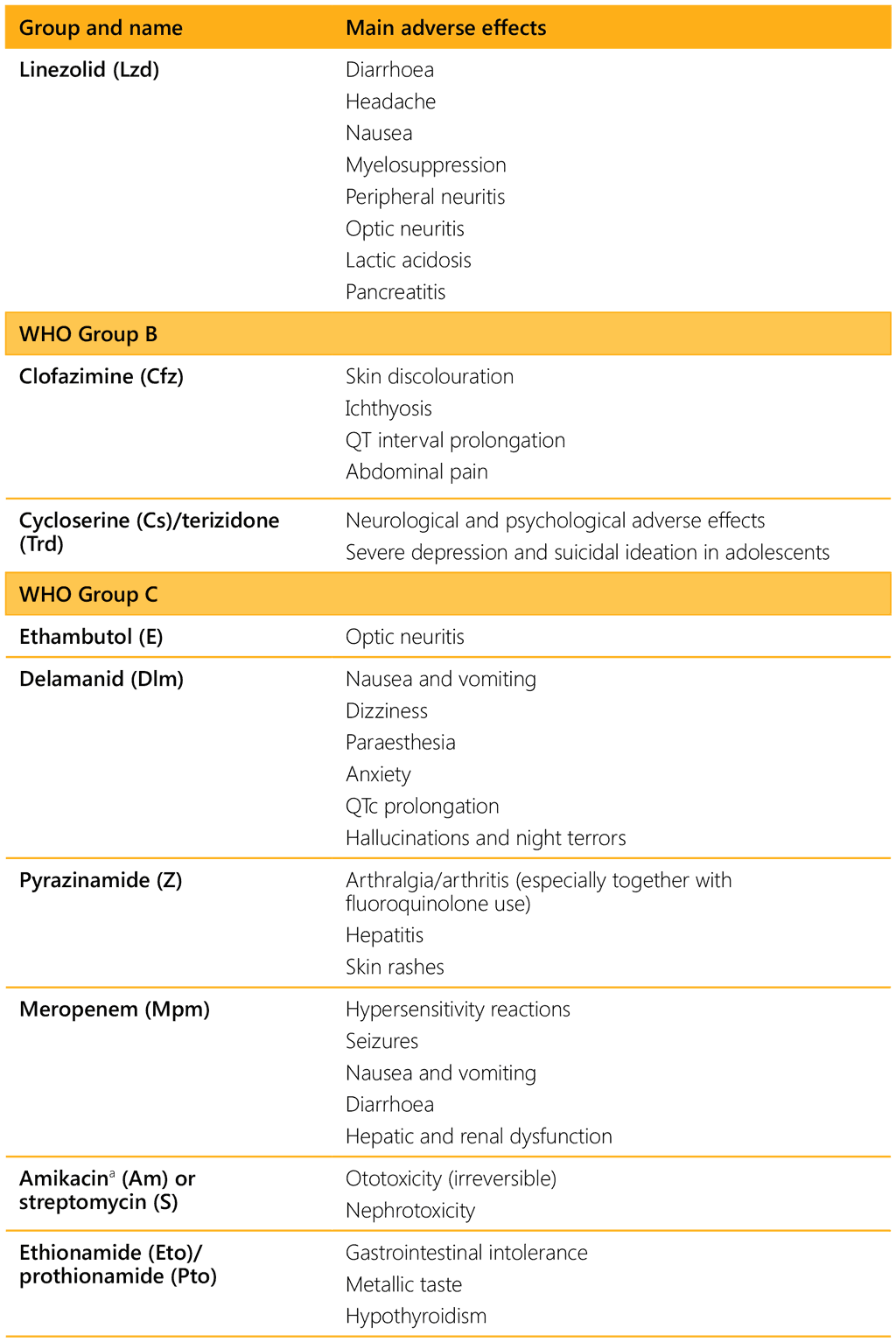
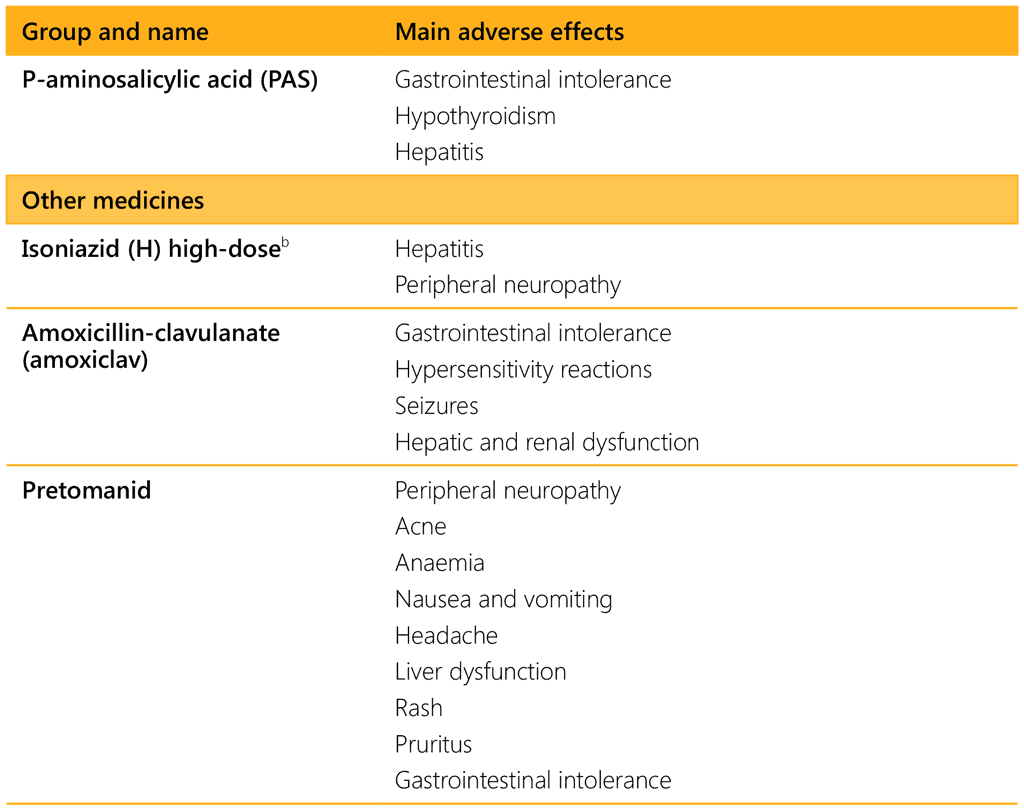
a Not recommended for use in children and adolescents aged under 18 years.
b If isoniazid is used, supplement with pyridoxine (vitamin B6) in infants and adolescents, in malnourished children, in children living with HIV, and when high-dose isoniazid is used to prevent peripheral neuropathy.
Source: adapted from Schaaf HS, Thee S, van der Laan L, et al. Adverse effects of oral second-line antituberculosis drugs in children. Expert Opin Drug Saf. 2016;15(10):1369–1381 (124); and https://www.tballiance.org/sites/default/files/assets/Pretomanid_Full-Prescribing-Information.pdf.
Key monitoring considerations for common or important adverse effects of MDR/RR-TB in children are described below (125, 126). The principles of monitoring in adolescents are similar to adults.
Electrocardiogram monitoring
Patients receiving any combination of the potentially QT-prolonging medicines (clofazimine, bedaquiline, delamanid or fluoroquinolones) should have regular electrocardiogram (ECG) monitoring. Given the composition of currently recommended regimens, most people being treated for MDR/RR-TB will be receiving one or more of these medicines and will need ECG monitoring. The fluoroquinolones are also known to prolong the QT interval. The effect of levofloxacin is relatively minimal, and ECG monitoring is therefore not critical for levofloxacin. Moxifloxacin, however, has a greater QT-prolonging effect and ECG monitoring should be considered when administered with other QT-prolonging medicines.
Ideally, an ECG should be done at baseline, 2 weeks and 4 weeks, and then every 4 weeks while on treatment and additionally as clinically indicated. The risk of a severely elevated QT interval (QTcF ≥500 ms) does not appear to be high in children or adolescents (82). If frequent monitoring is not feasible, then measurement at baseline, 4 weeks, 8 weeks and 24 weeks and additionally as clinically indicated may be a reasonable approach. As some of these medicines have long half-lives, it may take weeks before the maximum effect on the QT interval is observed.
Calculation of the corrected QT interval in children should generally follow the same procedures as for adults. Bazett’s formula may overcorrect at high heart rates that are normal in young children (resulting in a spuriously high QTc interval), so Fridericia’s formula (QTcF)24 is preferred in children. The use of paediatric-sized chest leads in young children with very small chests may improve accuracy.
Management of QTcF prolongation in children should follow the same steps as in adults, with symptom assessment, repeat of the ECG, electrolyte assessment and replacement if relevant, nutritional assessment, checking thyroid functions (ethionamide and P-aminosalicylic acid), and review of other medicines and possible clinical conditions. QTcF over 450 ms is considered prolonged. QTcF of 500 ms
or over raises the risk of a potentially life-threatening arrhythmia, and strong consideration should be given to withholding potentially QT-prolonging medicines until the QT interval has improved or withdrawing the culprit medicine as needed (82).
Full blood count and differential white cell count
Bone marrow toxicity (anaemia, thrombocytopenia or neutropenia) is frequently observed in people treated with linezolid. It is dose- and duration-dependent, meaning the risk increases with higher exposures and longer treatment durations. The effect can be severe and progress rapidly. In a small prospective study of children with MDR/RR-TB, 10 of 17 treated with linezolid developed anaemia (59%), including 3 with grade 3 events and 2 with grade 4 events (127).
Ideally, a full blood count with a differential should be done before starting treatment, then every 2 weeks for the first 2 months, and then every 4 weeks while on linezolid. This approach helps identify adverse events early. As cytopenias can progress rapidly, a full blood count should be repeated weekly if there is a meaningful decrease (e.g. by one or more grades) in haemoglobin, platelet or neutrophil counts. Temporary interruption of linezolid may be needed for worsening cytopenias, including to
allow time for other causes to be assessed. Anaemia, thrombocytopenia and neutropenia are usually reversible after stopping linezolid. Linezolid may need to be discontinued permanently, especially if the child had severe cytopenias, but it can be restarted at a lower dose if it is a key medicine in the regimen.
Monitoring for other toxicities related to linezolid is also important, including for peripheral neuropathy and optic neuritis. Testing reflexes at baseline or undertaking regular pinprick testing can be done to monitor for toxicities. Testing visual acuity in children at baseline and throughout treatment using symbol charts or “tumbling Es” can be done. For infants and toddlers, visual tracking of objects such as a finger or small toy can be used.
Neuropsychiatric side-effects
Monitoring for neuropsychiatric adverse events, including hallucinations, is important in children treated with medicines that have known neuropsychiatric side-effects, such as delamanid and cycloserine. These events should be reported through the national pharmacovigilance system.
Thyroid function testing
Hypothyroidism is a frequent adverse effect of ethionamide, prothionamide and P-aminosalicylic acid. The risk is higher when P-aminosalicylic acid is combined with ethionamide or prothionamide (124). Signs and symptoms of hypothyroidism are nonspecific and may be difficult to assess in young children. Hypothyroidism may have a negative impact on neurodevelopment in young children. It is important to perform regular (every 2 months) laboratory monitoring of thyroid function in all children
taking any of these medicines until they have been stopped, and to supplement with levothyroxine if there is clinical or laboratory evidence of hypothyroidism.
Assessments for hepatotoxicity
Ideally, children should have ALT with or without AST and bilirubin levels measured at baseline. One possible approach to monitoring for hepatotoxicity is to repeat at least ALT every 4 weeks (monthly) for the first 6 months, every 8 weeks thereafter, and additionally as clinically indicated. Clinical indications for liver function testing (at least ALT) are new-onset vomiting (starting after the patient is stabilized on treatment, even if only a few episodes), abdominal pain or tenderness (especially over the right
upper quadrant) and jaundice.
If the patient is jaundiced or has raised bilirubin levels and raised ALT, or there are clinical symptoms with ALT more than three times normal levels, or the patient is asymptomatic with ALT more than five times normal levels, all hepatotoxic medicines should be discontinued immediately. Other possible reasons for hepatitis (e.g. hepatitis A, B or C) should be excluded. Normalization of liver enzymes should be awaited. If clinically indicated, hepatotoxic TB medicines can then be reintroduced carefully
one by one. If the regimen is not failing, alternative TB medicines may be considered to replace the hepatotoxic medicines.
Social support and adherence measures
WHO recommends providing health education and counselling on the disease and treatment adherence to all people on TB treatment. Adherence counselling and support for the child or adolescent and their family are a crucial part of effective care for MDR/RR-TB care. Strong social support, parental or family counselling, and close relationships with health care providers help to improve care and treatment outcomes in children and adolescents. Children and adolescents should be encouraged to return to normal activities such as school and sports once they are clinically able to
handle these important daily activities and are no longer infectious (if bacteriologically confirmed at diagnosis). Recommended treatment adherence interventions include tracers or digital modification monitoring, material and psychological support to the patient or their family, and staff education.
Adolescents with TB may need additional counselling and support, especially if they have other comorbidities such as HIV. In addition to being at increased risk of developing MDR/RR-TB compared with adults, they may also be at risk for less favourable treatment outcomes than adults and younger children (128, 129). Medicines with adverse effects that affect appearance (e.g. clofazimine causing skin discolouration) may affect adherence in adolescents.


24 Fridericia’s formula: QTcF = QT/RR(0.33).
 Feedback
Feedback