Book traversal links for 5.5.2 Monitoring safety
Although the 9-month all-oral MDR/RR-TB regimen is taken for much less time than the longer regimens, this regimen still has a high pill burden and includes medications with multiple overlapping toxicities. The most common adverse events associated with the 9-month all-oral regimen are anaemia (among patients receiving the linezolid-containing regimen), hepatotoxicity, QT prolongation, nausea and vomiting (59). Treatment monitoring schedules must include relevant clinical and laboratory parameters to detect, manage and prevent common and significant adverse events in a timely manner.
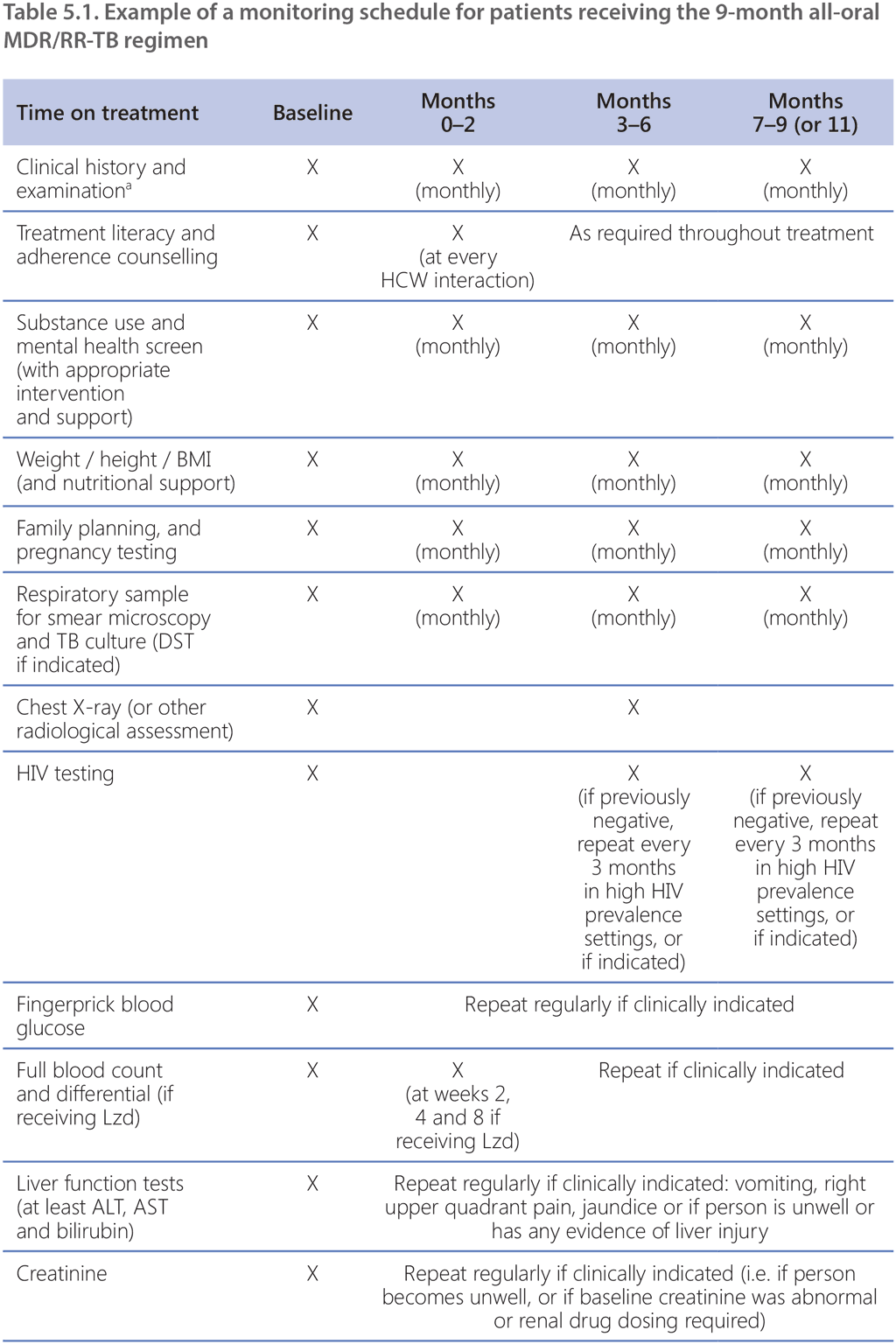
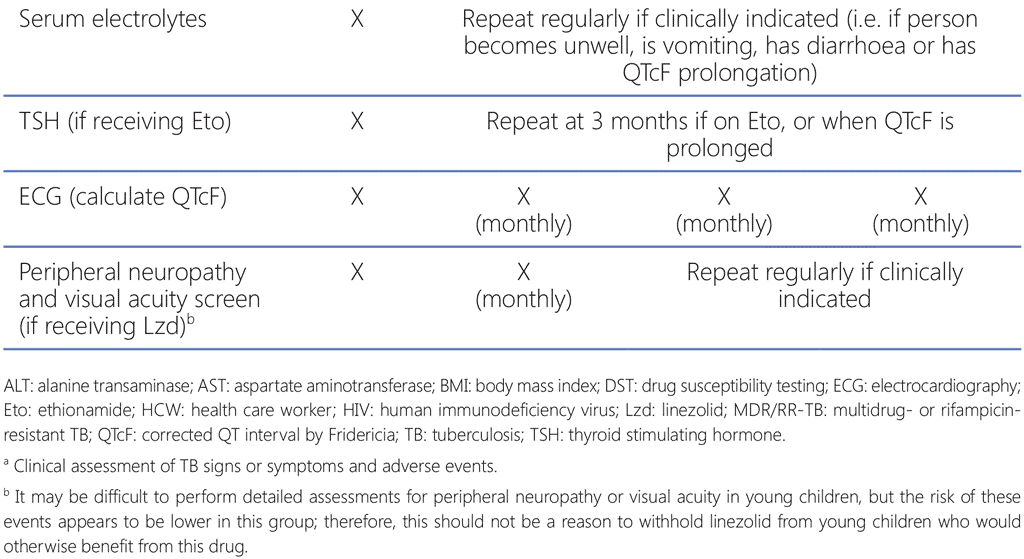
NTPs should already have established aDSM systems; aDSM involves the active and systematic clinical and laboratory assessment of patients on treatment with new TB medicines, or novel MDR/RR-TB regimens, to detect, manage and report suspected or confirmed drug toxicities (18). Adverse events (particularly those that are considered serious or severe, or lead to drug withdrawal) among patients receiving the 9-month all-oral regimen must be reported to the national agency responsible for pharmacovigilance, within the framework of aDSM. The WHO global aDSM database collects a standard set of variables and comprises anonymized IPD on adverse events from people treated with new TB medicines or novel MDR/RR-TB regimens (60). Countries where aDSM has been implemented are encouraged to contribute their national data to this database, to improve the collective knowledge of the safety of new TB medicines and regimens and to inform future TB treatment policies.
 Feedback
Feedback