Book traversal links for Annex 2: Conflict of interest assessment for Guideline Development Group and External Review Group members
Before being considered for group membership, each Guideline Development Group (GDG) and External Review Group candidate was required to submit a completed declaration of interest (DOI) form. In addition, a preliminary internet search was performed to identify any obvious public controversies or interests that may lead to compromising situations for the World Health Organization (WHO) and the expert concerned.
The candidate’s curriculum vitae (CV) and DOI, and information retrieved from the internet, were examined by steering committee members to assess whether there were, or may be, actual or perceived conflicts of interest and, if so, whether a management plan was required. This evaluation process, and resultant management plans, were based on the Guidelines for declaration of interests (WHO experts) (1) and the WHO handbook for guideline development (2nd edition) (2).
Both financial and non-financial interests were considered. A “significant” conflict of interest would include:
- “intellectual bias”, where an individual may have repeatedly and publicly taken a position on an issue under review, which may affect the individual’s objectivity and independence in the global policy development process;
- involvement in research or publication of materials related to issues under review; and
- a financial interest above US$ 5000.
Developers of any assay are never involved in the process of policy development; this is automatically considered a conflict of interest.
Once a determination was made that either no conflict of interest existed, or any conflict of interest could be appropriately managed, and a decision had been made to appoint the candidate, the name and a brief biography of each candidate were published on the WHO website for at least 14 days before the meeting, for public notice and comment.
DOI statements are summarized by the WHO steering committee at the start of the meeting. Selected individuals with intellectual or research involvement were invited as technical resource persons to provide technical input and answer technical questions. These individuals did not participate in the Grading of Recommendations Assessment, Development and Evaluation (GRADE) evaluation process and were excluded from the group discussions when recommendations were developed.
Table A.2.1. Summary of the declarations of interest statements for the GDG members: “Molecular assays intended as initial tests”, 7–18 December 2020
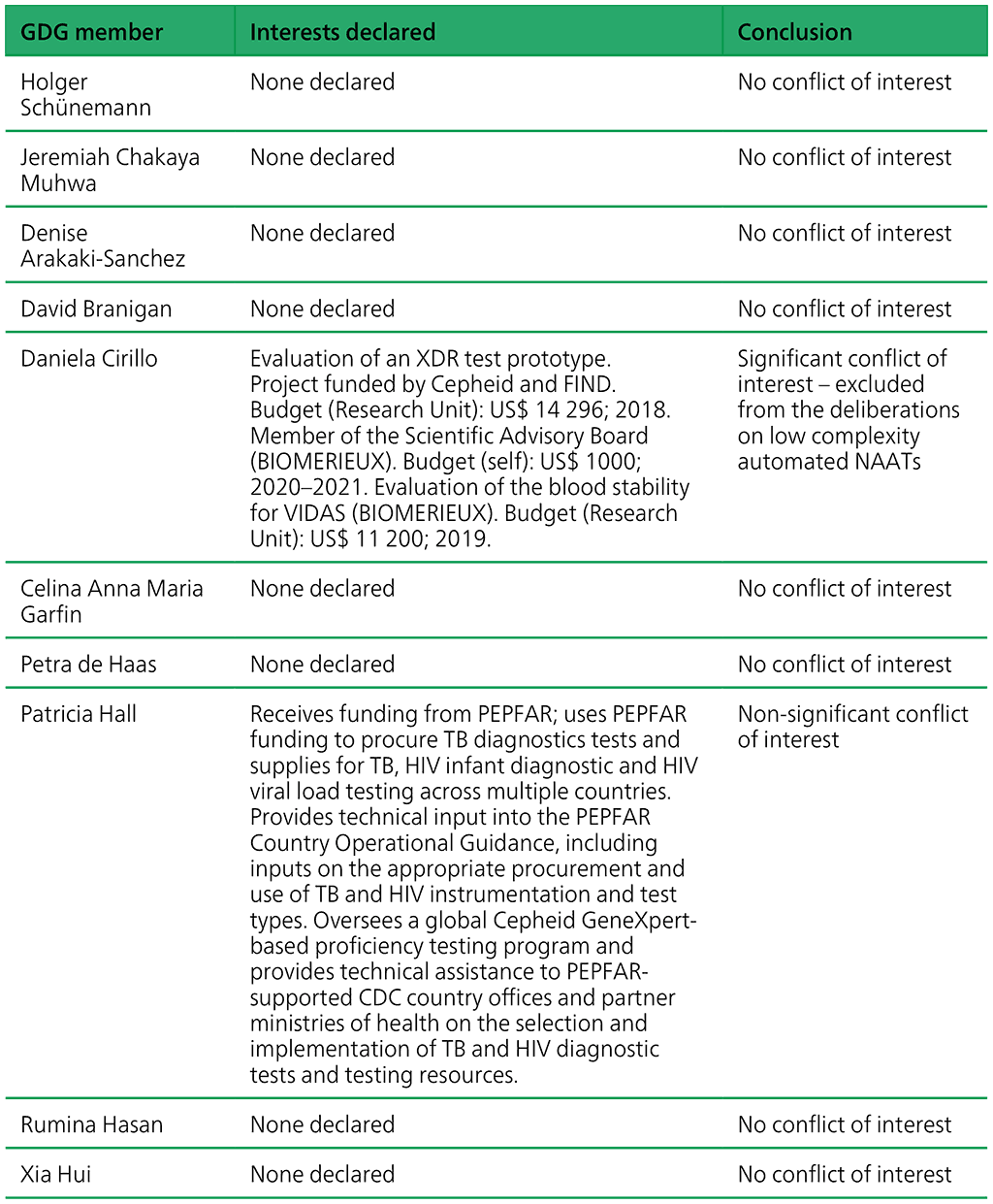
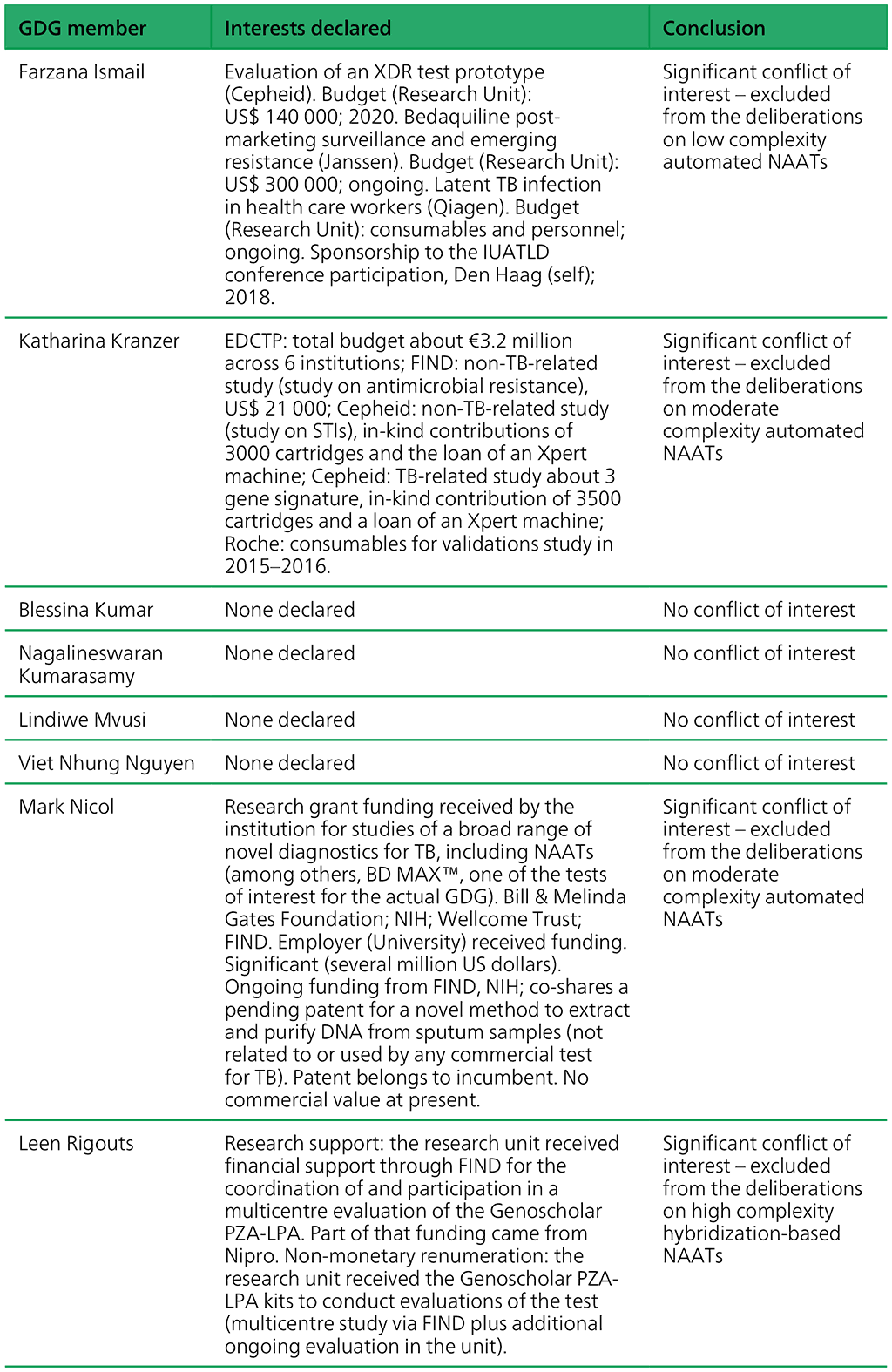
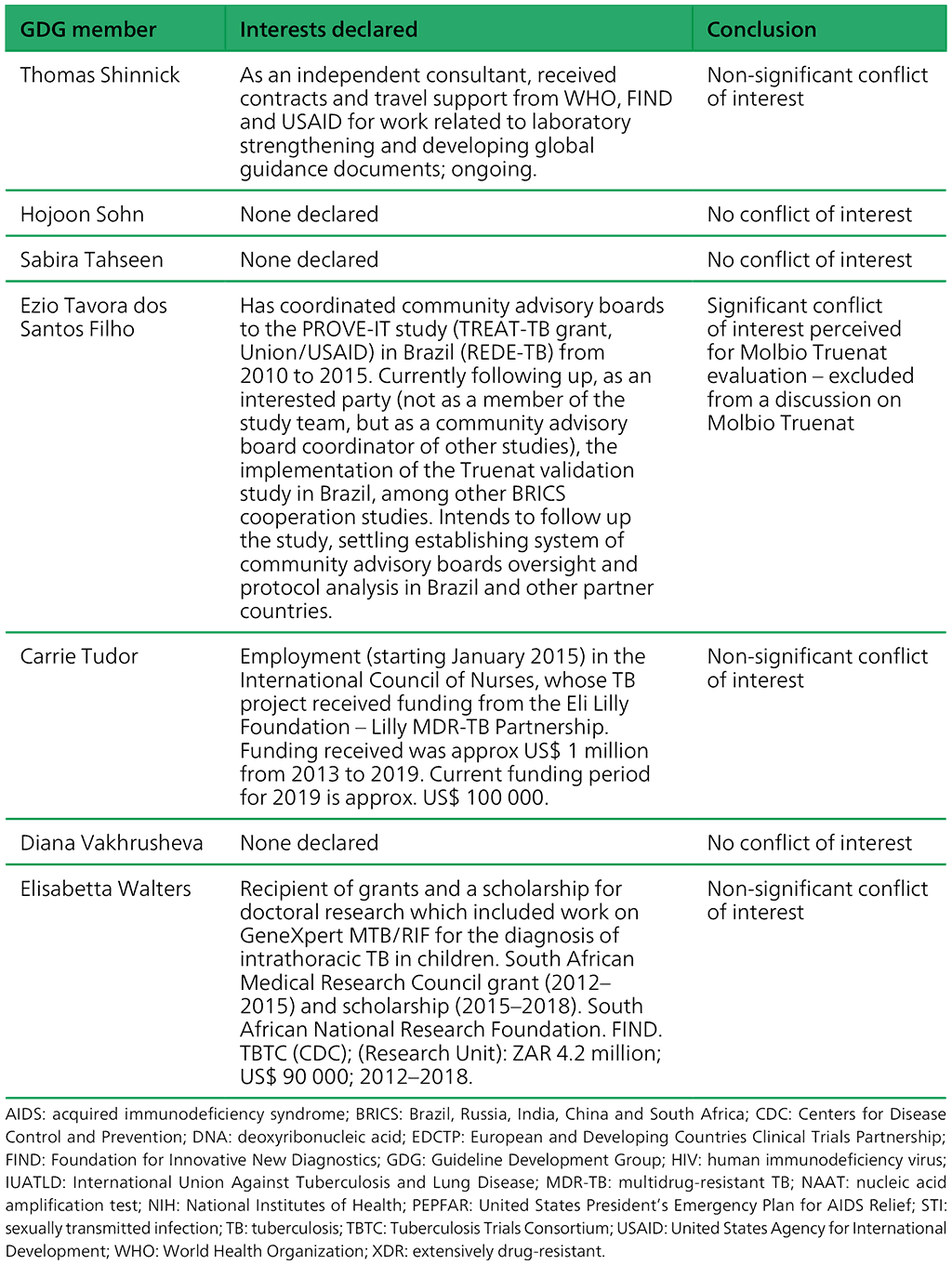
Table A.2.2. Summary of the declarations of interest statements for the ERG members: “Molecular assays intended as initial tests”, 7–18 December 2020
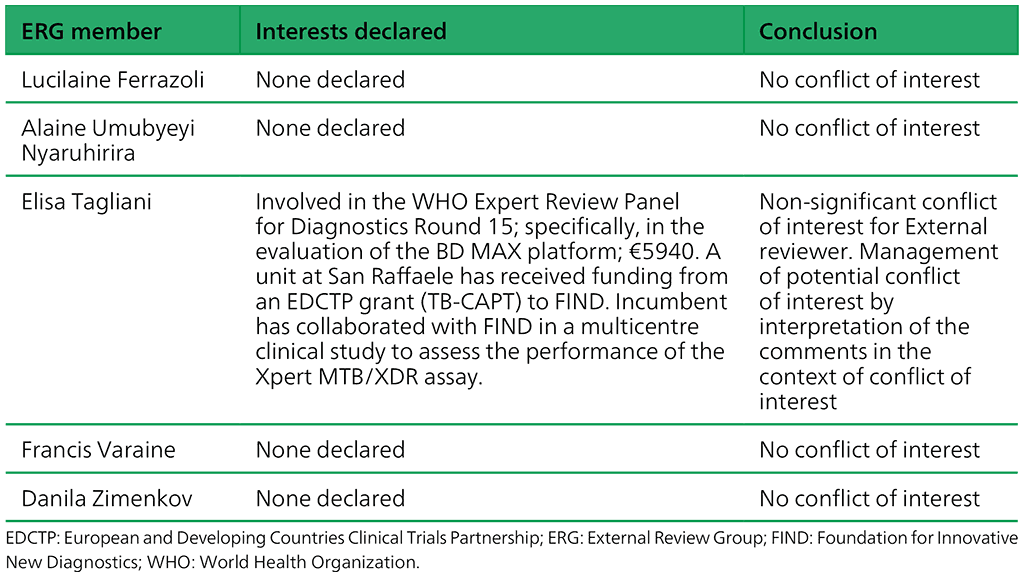
Table A.2.3. Summary of the declarations of interest statements for the GDG: “Targeted next-generation sequencing” 2–5 May 2023
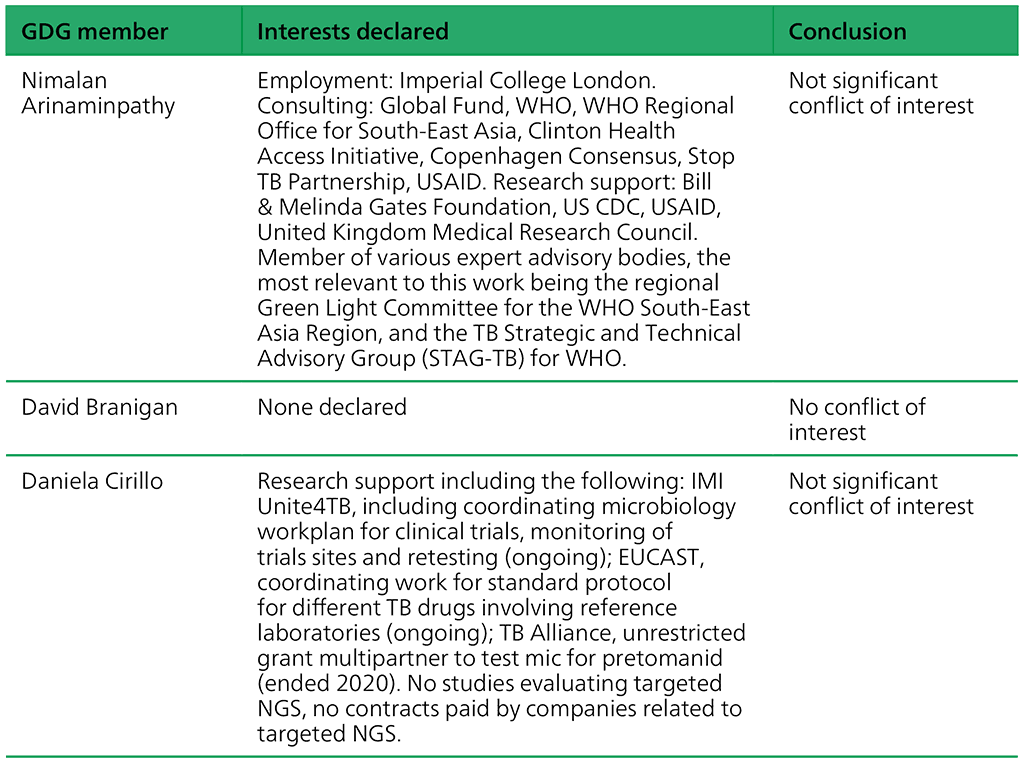
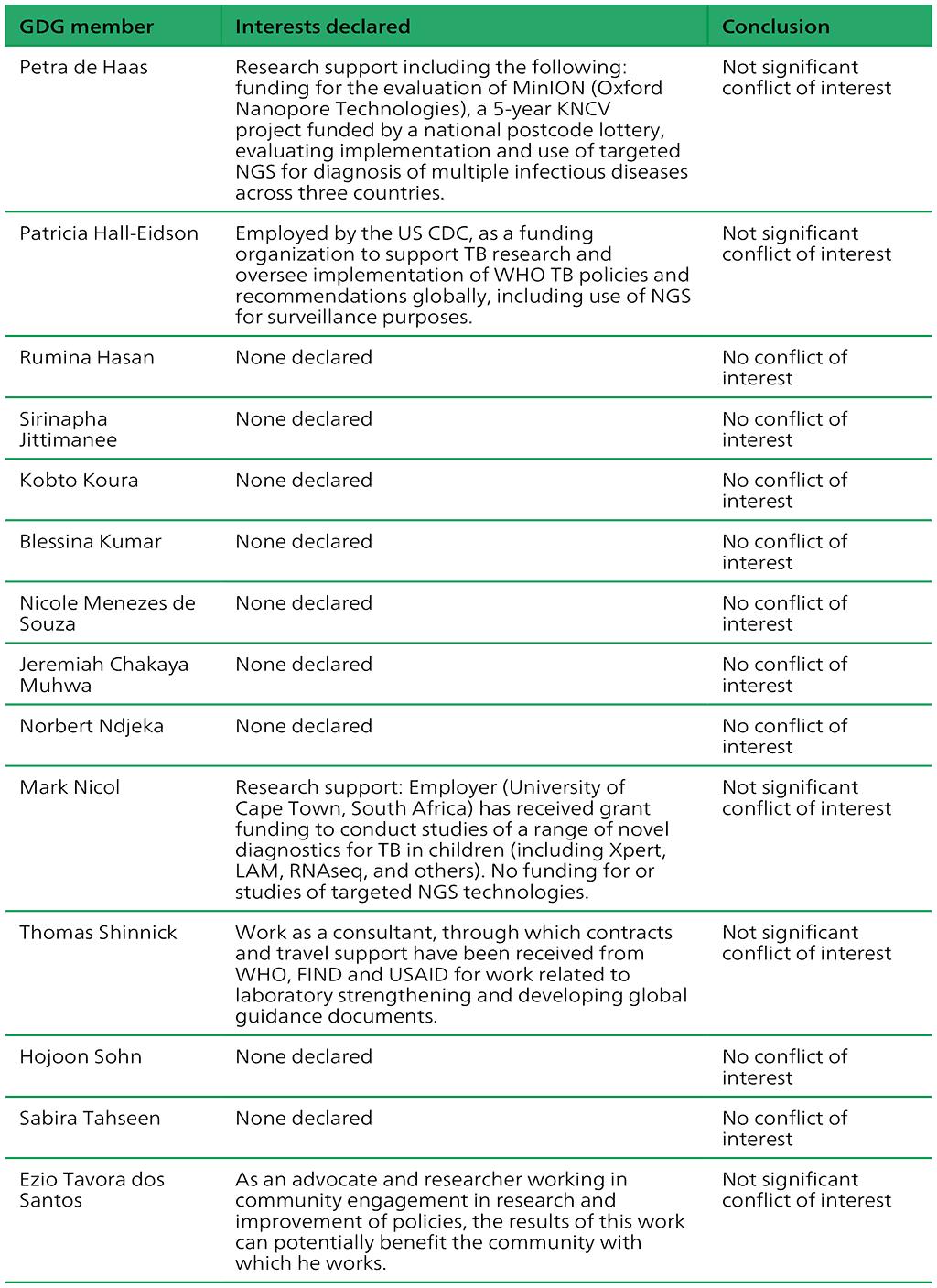
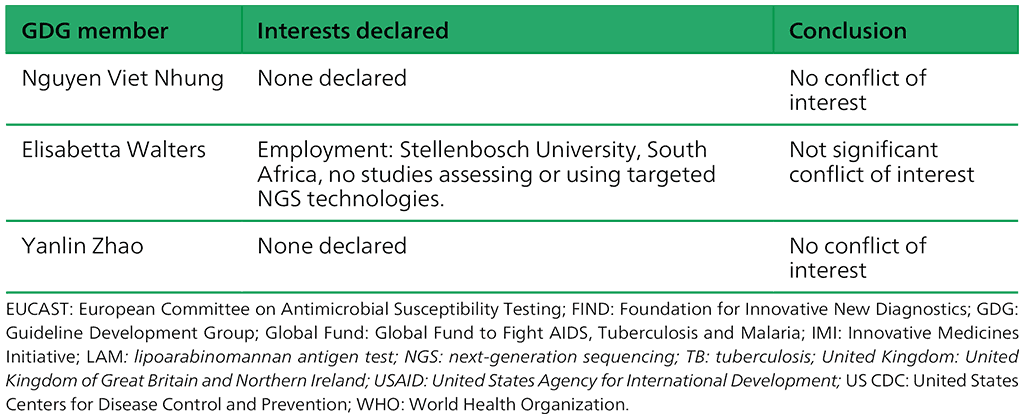
Table A.2.4. Summary of the declarations of interest statements for the ERG: “Targeted Next-Generation Sequencing” 2–5 May 2023
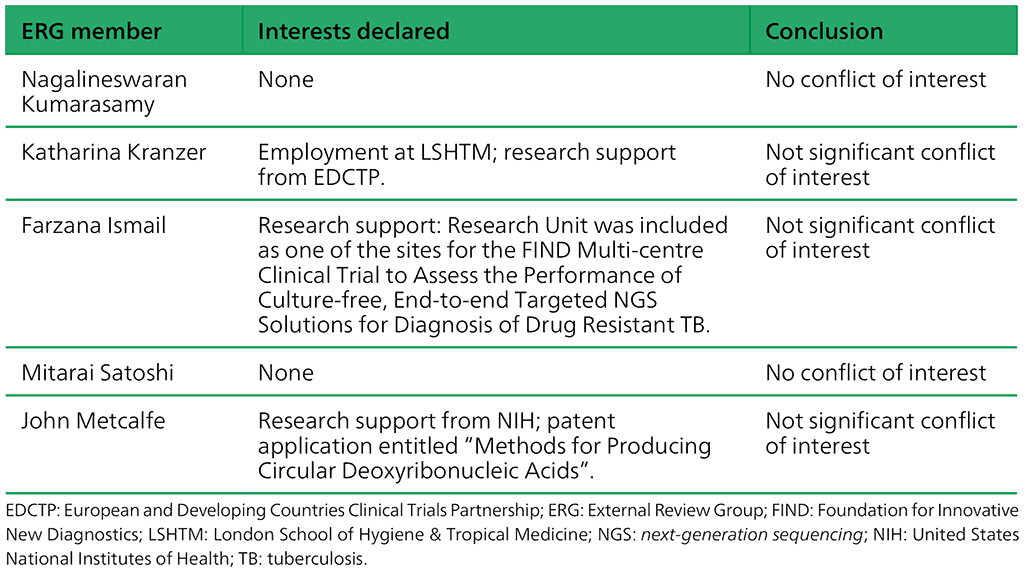
Before being invited to be a GDG member, each potential GDG member is asked to submit a completed declaration of interests (DOI) form and provide a CV. In addition, an abbreviated and focused internet search is performed “to identify any obvious public controversies or interests that may lead to compromising situations for WHO and the expert concerned”. Members of the steering committee evaluate a potential member’s CV, DOI and information retrieved from the internet to determine whether there are, or may be, conflicts of interest (COI) and, if so, whether these require a management plan. COI management is based on the WHO guidelines for DOI for experts (1),one-on-one consultation with a member of the Ethics Team from the WHO Office of Compliance, Risk Management and Ethics, and the WHO handbook for guideline development.²²
Both financial and non-financial interests are considered. A “significant” COI would include:
- “intellectual bias”, when an individual may have repeatedly taken a public position on an issue under review, which may affect the individual’s objectivity and independence in the global policy development process;
- involvement in research or the publication of materials related to the issue under review; and
- financial interest above US$ 5000.
For obvious reasons, developers of any assay are never involved in the process of policy development.
References for Annex 2
- Declaration of interests [website]. Geneva: World Health Organization; 2023 (https://www.who.int/about/ethics/declarations-of-interest).
- Handbook for guideline development 2nd ed. Geneva: World Health Organization; 2014 (https://apps.who.int/iris/handle/10665/145714).
22 WHO handbook for guideline development. Geneva: World Health Organization; 2014 (https://www.who.int/publications/guidelines/handbook_2nd_ed.pdf?ua=1, accessed 12 June 2020).
 Feedback
Feedback