Book traversal links for 4.6 Diagnostic approaches: drug-resistant TB
The clinical presentation of DR-TB in a child or adolescent is similar to that of other forms of TB in a child or adolescent. When DR-TB is suspected, it is important to collect respiratory samples (stool, expectorated or induced sputum, NPA sample or gastric aspirate) for bacteriological confirmation by Xpert MTB/RIF or Xpert Ultra when possible. Truenat MTB or MTB Plus may also be used for sputum specimens. Xpert and
Truenat assays provide rapid information that indicates rifampicin resistance and therefore DR-TB. Culture-based phenotypic DST, rapid, low-complexity automated nucleic acid amplification tests (NAATs) and LPAs allow testing for susceptibility to a wide range of medicines (see Box 4.11).
More details regarding diagnostic tests for detection of DR-TB are found in the WHO operational handbook on tuberculosis. Module 3: diagnosis – rapid diagnostics for tuberculosis detection (76) and Module 4: treatment – drug-resistant TB treatment (82).
If there is known contact with a person with DR-TB, it is important to obtain the resistance pattern of the most likely source case to guide treatment (see Section 5.3). There is over 80% concordance between DST patterns in children with TB and the likely source case (83). If this information cannot be obtained, treatment should be started based on the most likely drug susceptibility pattern.
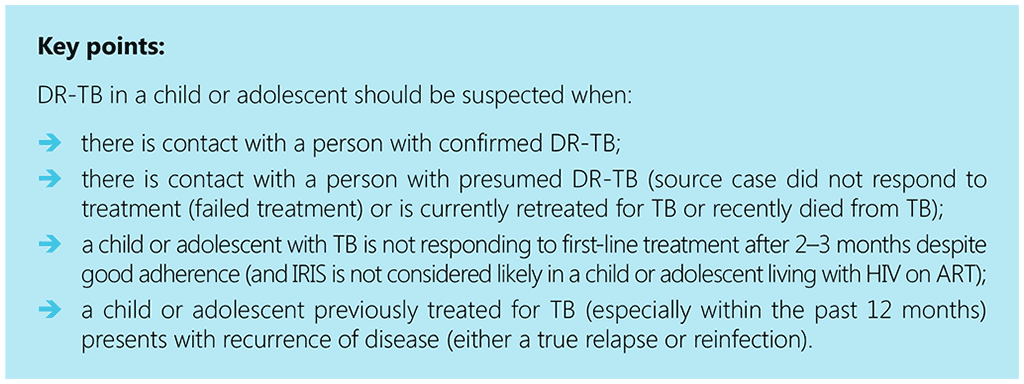
In all children aged under 10 years in whom a decision to treat for TB has been made based on the treatment decision algorithms, an assessment needs to be made for risk factors of DR-TB.
A high index of suspicion is required in children in close contact with a person with confirmed DR-TB and in children who have not improved clinically after completion of the intensive phase of first-line treatment (no improvement in symptoms, failure to gain weight, or persistently positive smear or culture). If a child is in close contact with a person whose TB treatment has failed, is non-adherent to TB treatment or has died from TB, information regarding confirmation of DR-TB in this person should be obtained (6, 72, 84).

 Feedback
Feedback