Book traversal links for 3.3 Regimen options in the treatment of DR-TB
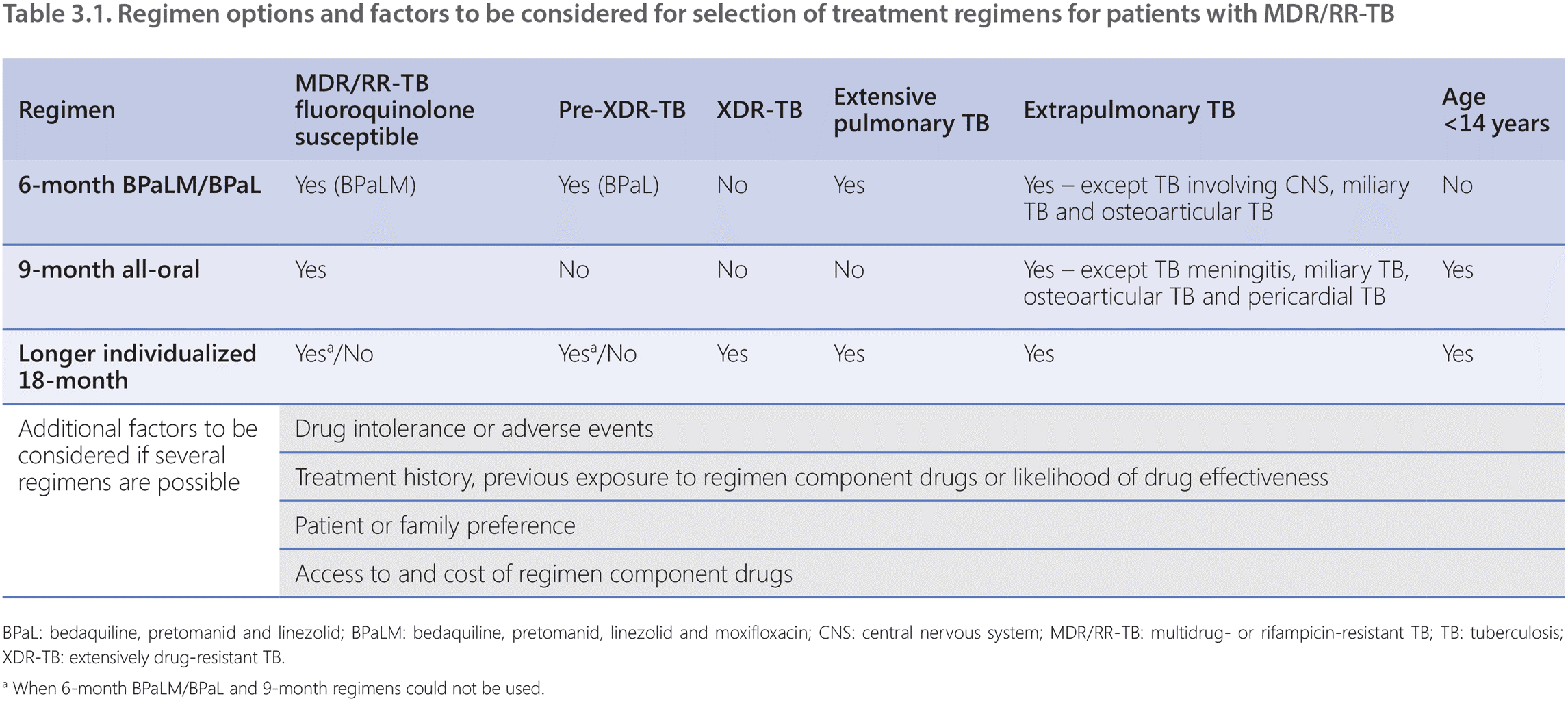
In patients with MDR/RR-TB, there are several regimens that can be used based on current WHO policy. The key factors that define treatment regimen choice include drug-resistance profile, prior exposure to TB medicines and patient history, drug-resistance profile of close contacts, the patient’s age, extent of pulmonary TB disease and localization of extrapulmonary TB lesions.
- BPaLM regimen (6 Bdq-Pa-Lzd-Mfx1): in patients with MDR/RR-TB where fluoroquinolone susceptibility is presumed or documented. This 6-month all-oral treatment regimen comprises bedaquiline, pretomanid, linezolid and moxifloxacin. It is possible to omit moxifloxacin and continue with the BPaL regimen for MDR/RR-TB patients with confirmed fluoroquinolone resistance.
- 9-month all-oral regimen (4–6 Bdq(6 m)-Lfx/Mfx-Cfz-Z-E-Hh-Eto or Lzd(2 m) / 5 Lfx/Mfx- Cfz-Z-E): in patients with MDR/RR-TB and in whom resistance to fluoroquinolones has been excluded. The 9-month all-oral regimen comprises bedaquiline (used for 6 months), in combination with levofloxacin/moxifloxacin, ethionamide, ethambutol, isoniazid (high dose), pyrazinamide and clofazimine (for 4 months, with the possibility of extending to 6 months if the patient remains sputum smear positive at the end of 4 months); followed by treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide (for 5 months). Ethionamide can be replaced by 2 months of linezolid.
- Longer individualized regimens: for patients with MDR/RR-TB who are not eligible for or had no favourable treatment outcome using the above 6-month or 9-month regimens, have TB disease caused by M. tuberculosis strains with extensive drug resistance (e.g. extensively drug-resistant TB [XDR-TB]) or have intolerance to key components of the above-mentioned regimens. These regimens have a duration of at least 18 months and are individually designed based on a hierarchical grouping of second-line TB medicines, the drug-resistance profile and the patient’s medical history.
Based on the review of the latest available evidence, the 6-month BPaLM regimen is the preferred option for most patients with MDR/RR-TB.
BPaLM is the regimen of choice for patients with MDR/RR-TB with absent or unknown fluoroquinolone resistance. In cases where resistance to fluoroquinolones is identified before or after treatment initiation, moxifloxacin can be omitted and the BPaL regimen without moxifloxacin should be initiated or continued, because there is probably no added benefit of using a drug with demonstrated resistance that may have toxicities. If resistance to bedaquiline, linezolid or pretomanid is confirmed or suspected, the BPaLM/BPaL regimen should be stopped, and patients should be referred for a longer individualized regimen.
The duration of BPaLM regimen is largely standardized for 6 months (26 weeks), whereas BPaL can be extended to a total of 9 months (39 weeks), as described in Chapter 4.
Patients with MDR/RR-TB who are aged below 14 years or who are pregnant or breastfeeding are not eligible for BPaLM and they will benefit from the 9-month all-oral regimens, composed of bedaquiline, levofloxacin/moxifloxacin, clofazimine, ethionamide or linezolid, ethambutol, isoniazid (high dose) and pyrazinamide. This regimen remains a treatment option for patients with MDR/RR-TB without fluoroquinolone resistance, who do not have extensive pulmonary TB disease or severe extrapulmonary TB, and patients with less than 1 month exposure to bedaquiline, fluoroquinolones, ethionamide, linezolid and clofazimine. When exposure is greater than 1 month, these patients may still receive this regimen if resistance to the specific medicines with such exposure has been ruled out.
The 9-month all-oral regimen has two variations, with either ethionamide or linezolid; however, in both variations, an initial phase comprises seven drugs (bedaquiline – given for 6 months, levofloxacin/moxifloxacin, clofazimine, ethionamide or linezolid), ethambutol, isoniazid (high dose) and pyrazinamide, followed by 5 months of treatment with levofloxacin/moxifloxacin, clofazimine, ethambutol and pyrazinamide. The initial phase usually lasts for 4 months, with the possibility of extending to 6 months if the patient’s sputum remains bacteriologically positive at the end of the fourth month. However, linezolid is used for only 2 months regardless of the duration of the first phase, whereas ethionamide should be continued until the end of the initial phase. Bedaquiline is used for the initial 6 months of the 9-month all-oral regimen but can be extended for longer under certain circumstances. Several eligibility criteria must be considered for this regimen, with additional considerations for the use of linezolid instead of ethionamide (Chapter 5).
The use of longer regimens is reserved for patients with MDR/RR-TB who are not eligible for the 6-month or 9-month regimen, patients in whom these regimens failed or patients with MDR/RR-TB with fluoroquinolone resistance and additional resistance to Group A medicines (XDR-TB). These patients would then receive a longer regimen designed using the WHO priority grouping of medicines recommended in the WHO consolidated guidelines (Chapter 6).
Decisions on appropriate regimens should be made based on likely efficacy, safety, patient preference and clinical judgement, also taking into account the results of susceptibility testing, patient treatment history, age, severity and site of the disease (Table 3.1).
1 The regimen notations used throughout this document highlight the number of months for which a relevant combination of medicines is used; where certain drugs are used for a different duration this is also noted, using subscript in brackets.
 Feedback
Feedback